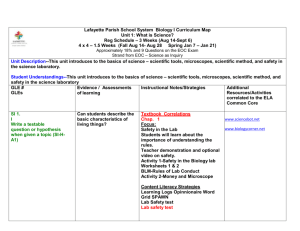
Sumner School District Scope and Sequence for Year
advertisement
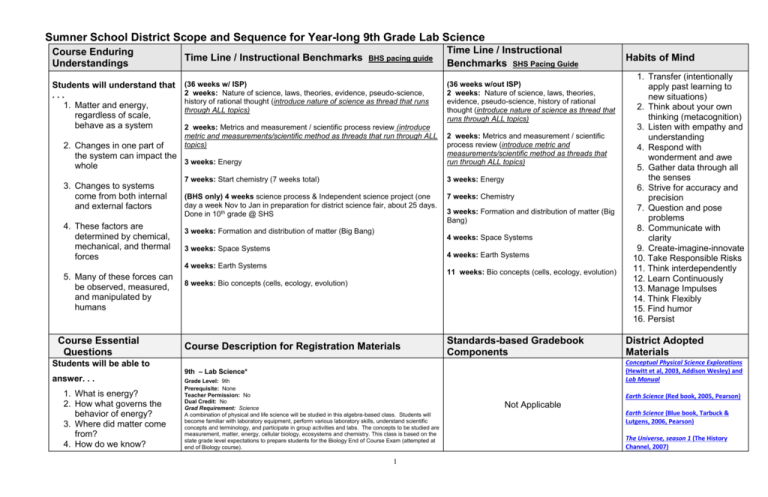
Sumner School District Scope and Sequence for Year-long 9th Grade Lab Science Course Enduring Understandings Students will understand that ... 1. Matter and energy, regardless of scale, behave as a system Time Line / Instructional Benchmarks BHS pacing guide (36 weeks w/ ISP) 2 weeks: Nature of science, laws, theories, evidence, pseudo-science, history of rational thought (introduce nature of science as thread that runs through ALL topics) 2 weeks: Metrics and measurement / scientific process review (introduce metric and measurements/scientific method as threads that run through ALL topics) 2. Changes in one part of the system can impact the 3 weeks: Energy whole 3. Changes to systems come from both internal and external factors 4. These factors are determined by chemical, mechanical, and thermal forces Course Essential Questions Students will be able to answer. . . 1. What is energy? 2. How what governs the behavior of energy? 3. Where did matter come from? 4. How do we know? (36 weeks w/out ISP) 2 weeks: Nature of science, laws, theories, evidence, pseudo-science, history of rational thought (introduce nature of science as thread that runs through ALL topics) 2 weeks: Metrics and measurement / scientific process review (introduce metric and measurements/scientific method as threads that run through ALL topics) 7 weeks: Start chemistry (7 weeks total) 3 weeks: Energy (BHS only) 4 weeks science process & Independent science project (one day a week Nov to Jan in preparation for district science fair, about 25 days. Done in 10th grade @ SHS 7 weeks: Chemistry 3 weeks: Formation and distribution of matter (Big Bang) 3 weeks: Formation and distribution of matter (Big Bang) 4 weeks: Space Systems 3 weeks: Space Systems 4 weeks: Earth Systems 4 weeks: Earth Systems 5. Many of these forces can be observed, measured, and manipulated by humans Time Line / Instructional Benchmarks SHS Pacing Guide 11 weeks: Bio concepts (cells, ecology, evolution) 8 weeks: Bio concepts (cells, ecology, evolution) Course Description for Registration Materials Standards-based Gradebook Components 1 1. Transfer (intentionally apply past learning to new situations) 2. Think about your own thinking (metacognition) 3. Listen with empathy and understanding 4. Respond with wonderment and awe 5. Gather data through all the senses 6. Strive for accuracy and precision 7. Question and pose problems 8. Communicate with clarity 9. Create-imagine-innovate 10. Take Responsible Risks 11. Think interdependently 12. Learn Continuously 13. Manage Impulses 14. Think Flexibly 15. Find humor 16. Persist District Adopted Materials Conceptual Physical Science Explorations (Hewitt et al, 2003, Addison Wesley) and Lab Manual 9th – Lab Science* Grade Level: 9th Prerequisite: None Teacher Permission: No Dual Credit: No Grad Requirement: Science A combination of physical and life science will be studied in this algebra-based class. Students will become familiar with laboratory equipment, perform various laboratory skills, understand scientific concepts and terminology, and participate in group activities and labs. The concepts to be studied are measurement, matter, energy, cellular biology, ecosystems and chemistry. This class is based on the state grade level expectations to prepare students for the Biology End of Course Exam (attempted at end of Biology course). Habits of Mind Earth Science (Red book, 2005, Pearson) Not Applicable Earth Science (Blue book, Tarbuck & Lutgens, 2006, Pearson) The Universe, season 1 (The History Channel, 2007) 5. How does matter change? 6. What determines how matter behaves? 7. How does energy move through living systems? Ron Thompson’s 8 volume Life Science curriculum supplement Page Keeley, Uncovering Student Ideas in Life Sciences National Science Digital Library Atlas of Science Literacy Nature of Science Unit Sequence: 2 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) 9-12 INQA Question Scientists generate and evaluate questions to investigate the natural world. 9-12 INQC Explain Conclusions must be logical, based on evidence, and consistent with prior established knowledge. “What questions does the work in this unit answer?” (Unit Guiding Questions) 1. 2. 9-12 INQE Model 9-12 INQF Communicate 9-12 INQG Intellectual Honesty 9-12 INQH Intellectual Honesty 3. The essence of scientific investigation involves the development of a theory or conceptual model that can generate testable predictions. Science is a human endeavor that involves logical reasoning and creativity and entails the testing, revision, and occasional discarding of theories as new evidence comes to light. 4. 5. Scientists carefully evaluate sources of information for reliability before using that information. When referring to the ideas or findings of others, they cite their sources of information. What is the Nature of Science? (True science vs. Pseudo-science) What are the differences between laws, facts, theories, and hypotheses? What is a theory and how does it change in relationship to new evidence? How do we ensure validity in science? How do we know that what we are testing is what we want to be testing (e.g. appropriate use of experimental groups to increase accuracy)? How do we enhance reliability in science? How do we know that our results are reliable (repeated and consistent trials to increase precision)? How do we support conclusions with specific and reliable experimental evidence? Public communication among scientists is an essential aspect of research. Scientists evaluate the validity of one another’s investigations, check the reliability of results, and explain inconsistencies in findings. 2 “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) Assessments: *Making Conclusions based on Evidence vs. Inference *Evidence vs. Inference Quiz *Nature of Science Assessment “What will we have them do to learn and practice it?” (Instructional Activities) *Is it a Theory? probe (Keely, Vol. 3, pg 83) *Doing Science (Keely, Vol. 3, pg.93 *What is a Hypothesis? (Keely, Vol. 3, pg 101) *Nature of ScienceT or F PowerPoint probe *Nature of Science and Pesudo-science Worksheet *Vocab Carouselrotate around room practicing vocab *Pair and Share Bad Science Scenarios “What learning and practice materials are needed?” (Materials) Physical Science and Nature of Science Assessment Probes, Page Keely, Volume 3 Nature of Science PowerPoint Probe Nature of Science Common Assessment Physical Science Explorations, Hewitt 2003 Chapter 1 pages 4-12 References: Pseudosciencehttp://www.chem1.com/acad/sci/pseudosci.html Measurement Unit Sequence: 2 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) 9-12 INQB Scientific progress requires the use of various methods appropriate for answering different kinds of research questions, a thoughtful plan for gathering data needed to answer the question, and care in collecting, analyzing, and displaying the data. 9-12 INQD The methods and procedures that scientists use to obtain evidence must Communicate be clearly reported to enhance opportunities for further investigation. Clearly 9-12 APPD The ability to solve problems is greatly enhanced by use of mathematics and information technologies. “What questions does the work in this unit answer?” (Unit Guiding Questions) 1. What are the standard units of measurement in the metric system? 2. How do we convert within and between units? 3. How do we collect applicable, accurate, and reliable data? 4. How do we analyze and display experimental data? 5. What tools do we use to measure scientific data? “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) Metric Common Assessment “What will we have them do to learn and practice it?” (Instructional Activities) “What learning and practice materials are needed?” (Materials) scale / comparison to objects unit conversion review most common metric measurement units work measurement into all assessments Thompson’s Inquiry Series: The Metric System, Interpreting Graphs and Graphing Data (probe) Energy Unit Sequence: 3 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) 9-11 PS3A Although energy can be transferred from one object to another and can be transformed from one form of energy to another form, the total energy in a closed system remains the same. The concept of conservation of energy, applies to all physical and chemical changes. 9-11 PS3B Kinetic energy is the energy of motion. The kinetic energy of an object is defined by the equation: Ek = 1/2 mv2 9-11 PS3C Gravitational potential energy is due to the separation of mutually attracting masses. Transformations can occur between gravitational potential energy and kinetic energy, but the total amount of energy remains constant. “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) “What questions does the work in this unit answer?” (Unit Guiding Questions) Transfer Transformation Conservation 1. What are the 5 main forms of energy? (chemical, thermal, mechanical, nuclear, electromagnetic) 2. What is the definition of energy? (the ability to do work) 3. What is the difference between an energy transfer and an energy transformation? 4. What are the two states of energy? (Kinetic or Potential) 3 Pre-assessment (8th grade energy common assessments) “What will we have them do to learn and practice it?” (Instructional Activities) Qualitative observation of practical physics labs to understand / emphasize concept of energy transfers (do include mass and distance calculations) Student designed experiments with individual questions (CPO materials at BHS) “What learning and practice materials are needed?” (Materials) Pruett’s energy category activity--NEED project For example of energy transformation: http://www.inhabitots.com/energy-generatingsoccer-ball-brings-power-to-developingnations/ Physical science text, chapter 6, introduction to energy chapter 9, heat / thermal energy Keeley,USI in Physical Science, probe #12 , p. 59 5. How does energy transform from one type to another? 6. What is the conservation of energy? 8th: energy in / out Newton’s laws radiation (personalizing science-impacts of nuclear energy) survey of renewable & non-renewable energy sources--fracking (pro-con) 4 Chemistry Unit Sequence (7 weeks) “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) “What questions does the work in this unit answer?” (Unit Guiding Questions) “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) “What will we have them do to learn and practice it?” (Instructional Activities) 1. PS2B Atoms of the same element have the same number of protons. The number and arrangement of electrons determines how the atom interacts with other atoms to form molecules and ionic crystals. What is matter? Atomic Structure Probe What are the properties of matter? Atomic Structure and Periodic Table Practice Quiz (open note) 2. 3. What governs the behavior of matter? PS2A Atoms are composed of protons, neutrons, and electrons. The nucleus of an atom takes up very little of the atom's volume but makes up almost all of the mass. The nucleus contains protons and neutrons, which are much more massive than the electrons surrounding the nucleus. Protons have a positive charge, electrons are negative in charge, and neutrons have no net charge. PS2C When elements are listed in order according to the number of protons, repeating patterns of physical and chemical properties identify families of elements with similar properties. This Periodic Table is a consequence of the repeating pattern of outermost electrons. Bio Teacher Requests: add LS1F Food molecules PS2F All forms of life are composed of large molecules that contain carbon. Carbon atoms bond to one another and other elements by sharing electrons, forming covalent bonds. Stable molecules of carbon have four covalent bonds per carbon atom. (most likely fit Bio uint) PS2G Error! Hyperlink reference not valid.Error! Hyperlink reference not valid.Error! Hyperlink reference not valid.s change the arrangement of atoms in the molecules of substances. Chemical reactions release or acquire Error! Hyperlink reference not valid. from their surroundings and result in the formation of new substances (most likely fit Chem unit with potential review in Bio unit) Atomic structure & Periodic Table quiz What is the periodic table and how is it organized and used? 4. 5. How has our knowledge of matter developed over time? Physical versus chemical properties & changes Pure substances (Elements) compounds, molecules (only non metals can be a compound), mixtures Atomic structure and the behavior & properties of subatomic particles, history of the atom Definition & relevance of isotopes (exposure / intro level) Atomic number / atomic mass / mass number Ions Bonding (intro level—electrons shared, lost, gained. Leave covalent and ionic bonding and polarity for Bio) Periodic table—, groups & valence numbers, periods, families (1, 2, 16, 17, 18), metals, metalloids, non-metals, memorize first 20 elements Chemical reactions—products and reactants (law of conservation of matter) (include questions / concepts from previous tests on subsequent ones) 6. 9th Chem Common Assessment #1 7. 9th Chem Common Assessment #2 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 5 1st 20 Elements Flash Cards: Name on one side, symbol and atomic # on the back Reading text supplement 17.1 Visualizing the Invisible (20 min) Oobleck Demo: Observation/Inferences, Imagine the invisible, States of Matter review (40 min) *Pop-Can implosion: Observe/Inference – imagine molecular movement, states of matter (gas) (40 min) Mixtures Lab: Markers separating (quick lab after substances vs. mixtures notes) (60 min) OR 22.3 Activity CPS Elements vs. Compounds discussion (flow chart) Physical and Chemical Properties/Changes – demos on 8 physical properties and changes; notes + you tube glass blowing; tie back to Ziploc bag (observing chemical reactions) Do the demo -- students brainstorm what the phy property is (60 min) Shape – wood cube vs wad of paper Color – penny vs. dime State of Matter – solid, liquid, gas – Density – penny vs cork in water Solubility – beaker, penny in there, food color (penny insoluble, food coloring soluble) Boiling Pt /Condensation/Freezing Pt/Melting Pt. -- beaker with ice, hold over burner, boils Conductivity/Electrical Conductivity – Bruce light bulb with 2 prongs with sugar and salt in water Conductivity of Heat – scoopula to hand or side of face, get it red hot, ask if willing to still touch hand/face Reading p. 356 CPS text; internet assignment about chemical and physical changes and states of matter (60 min) Lesson on how to read chemical formulas (Activity 21.2 Minds On Activity CPS) and Sage – practice and follow up opener; includes 5 rules to read aloud and put in their own words (60 min) Chemical Reactions (Changes) – Measuring Mass in a chemical reaction (CPS book Activity 21.3) plus Conservation of Mass (60 min) or Steel Wool Lab *Solutions Activity 22.2 CPS (talk again about physical properties) Research scientists for a day – Atomic Theory as bridge to atomic structure (60 min) Probe: Atomic structure 2-D draw of historical atomic models as homework after notes History of Chemistry video: has study guide with it (60 min) “What learning and practice materials are needed?” (Materials) Chemistry DVDs (23 min each, 1 5-DVD set with teacher’s guide purchased for each high school, Nov 2011) Atomic Structure History of the Periodic Table Compounds Elements Using the Periodic Table 20. 3-D models of 3 different atoms to show different sizes, 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. etc. plus placement of atomic subparticles – can show gauge Intro notes on atomic structure… base on pretest/probe 5-2 Structure of an Atom reading with questions (if necessary), atomic numbers worksheet Structure of Atoms Activity with gluing beans Isotopes (if not already introduced) Pick an element and create Pop-Up Ad to “Sell an Element” Alien Activity… (2 60 min. classes) History of Periodic Table DVD response sheet Set-up and Families of the Periodic Table Powerpoint with links (Notes on different families/structure of periodic table) Properties of Elements DVD (Compounds, groups, families, etc.) Alkali Metals Demo Properties of Elements Lab: Metals, Non-metals, Metalloids lab Alkaline Earth Metal Lab Bonding and Chemical Reactions (basics) Bohr Model Lab with boards and poker chips as review Also, density oil food coloring in grad cylinder demo (Rina) Formation of Matter Unit Sequence: 3 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) “What questions does the work in this unit answer?” (Unit Guiding Questions) Evolution of the universe ES1B—Big Bang, Red/Blue shifts, spreading of matter, element formation (fusion / fission) Bridging question from Chemistry: Where did matter come from? How did it get located / distributed the way it is? Unit questions: What evidence do we have for the Big Bang? How did the elements form? How do we know about what we can’t see? To what extent have scientists accounted for all the matter in the universe? “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) Beyond the Big Bang Quiz “What will we have them do to learn and practice it?” (Instructional Activities) 1. View video and answer advance 2. 3. 4. 5. 6. 7. 8. organizer questions Make observations of distribution of matter from expanding and popping balloons Read and discuss articles View picture reveal / demo of visual vs. entire light spectrum Take an instruction survey (pretest) Complete a wave length worksheet Generate and observe characteristics of waves in lab setting Experiment with how waves are either blocked by, or can penetrate, different materials 6 “What learning and practice materials are needed?” (Materials) 1. 2. 3. 4. 5. 6. 7. 8. 9. Beyond the Big Bang video w/ question guide (History Channel--Brian), Big Bang balloon labs—1st expansion (dots on outside), 2nd explosion (balloon w/ contents proportional to elements exploded--pop for distribution of matter) Cosmic Times articles Demo--covered picture / reveal to show how we only see a portion of what’s available if we don’t use all areas of the spectrum “Lighten-up” survey Wave length activity worksheet / Electro-magnetic spectrum (Brian) EM spectrum lab “Check out these waves” (tapping center of a pan of water at different rates—circular waves, slinkies—linear horizontal waves, defraction grating glasses) refraction (bending) vs. reflection (bouncing back) Invisible light sources and detectors lab (filter lab) remote control under document camera exposes the IR, wrap cell phone lab in different materials. (Include red shift / blue shift concept using slinkies and YouTube video red / blue shift as post-lab discussion) Flame lab (demo)--colors of elements as signature, Invisible Universe book (Lawrence Hall of Science, 2002, ISBN 0-924886-69-2), spectrum tubes, defraction grating glasses, spectroscopes Keeley, USI in Astronomy, probes #38-45 (NOT 42), pp. 203- 7 SPACE SYSTEMS UNIT SEQUENCE (universe, galaxy, star, solar system, planets, meteorites, comets): 4 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed-could be from State GLEs, National, or Program Certification Standards) PS2A, B, C—Periodic Table, Structure of an atom ES1A—star formation (big idea of gravity of attractive force), planet formation “What questions does the work in this unit answer?” (Unit Guiding Questions) “How will we know if they have learned it?” (Assessment Tasks-evidence of learning will come from . . .) Scale of the universe Formative Assessment probe: 1. Probe--List of universe structures, define (what is it?) and rank order by size. 2. Scale of Systems: Galaxies, Solar System/Stars, Planets “Tops Scale the Universe #44” ?, pp. 32-38 (activities), 3. Imax Cosmic Voyage (uses powers of 10 and is good for teaching scale) What was formed as the result of the Big Bang? Where do the first elements come from? How are galaxies and solar systems and formed? (adv: How does the combination of gravity and angular momentum affect matter?) How are stars formed and what happens inside of stars? (fusion / fission—bridge to chemistry, law of conservation of mass, mass is lost and must be accounted for—lost mass is converted to energy, matter is converted into energy, stellar evolution and matter formation) “What will we have them do to learn and practice it?” (Instructional Activities) Star Formation: captioned comic strip using 5 steps of star Star Formation: Fusion / Fission formation (use personification) 1. Interstellar nebula clumping due to gravity (Aspire Star life cycle site Space unit end common assessment How did the left over matter become different types of planets? How can the motion of planets be explained? http://aspire.cosmic-ray.org/labs/star_life/starlife_proto.html protostar web tour. Complete questions on protostar handout Students develop 6 panel comic strip with captions to explain sequence (answers to first 4 questions can be found by scrolling below the table of contents) 2. “Star bucks” Probe --Fusion / fission activity: using “element trading” cards 3. Galaxy Formation: Red book (chpt. 21) 4. Conceptual Physics book (chpt. 40) Reading pg. 725-731 5. Universe DVD series “Life cycle of a star” 6. (Townley) “Cosmic Chemistry” powerpoint including law of conservation of mass 7. Use Chandra posters and “Star life Guided Web Tour” webquest http://aspire.cosmic-ray.org/labs/star_life/starlife_main.html to teach life cycles of different sized stars (giant, huge, sun-like, brown dwarf) 8. Black hole gravitational pull demonstration: Pie pan, kyro syrup (1/2’) with food color, hole punches, and different sized balls, place in the center, turn in one direction, note changes as ball is turned 9. Star formation flow chart handout & power points 10. Students diagram out a large star cycle and small/ medium star cycle Solar system Formation: 1. Distance to the planets (Tacoma Astronomical Society)—requires a meter long strip of adding tape or 4” wide strip of paper towel per for students to write on. Planetary formation—angular momentum, tie to proto-star formation and remaining material and energy. Use student active demos to show attraction and spinning between objects 1. Have several students stand in places throughout the room. 2. Have one student start slowly spinning. 3. The student closest to them get draw to the spinning student. 4. Explain that as you gain more mass you increase gravity. 5. Then a student a little farther away is drawn in, repeat the steps Magnetic stir plate spinning demo. Use a beaker/Flask and stir plate. 1. Put water, spinner, and paper hole punches in the beaker/Flask. 2. Start to spin the spinner in the Beaker/Flask slowly. 3. Slowly increase the speed of the spinner. 8 “What learning and practice materials are needed?” (Materials) As you increase the speed of the spinner the dots will be drawn toward the middle Earth Science Red book reading 712-729 4. 9 EARTH SYSTEMS UNIT SEQUENCE: 4 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) “What questions does the work in this unit answer?” (Unit Guiding Questions) 9-11 ES2A Global climate differences result from the uneven heating of Earth's surface by the Sun. Seasonal climate variations are due to the tilt of Earth's axis with respect to the plane of Earth's nearly circular orbit around the Sun Bridge from space: 1. How did our Earth form? Why is it round? 2. What is the structure of the earth? 3. What creates an atmosphere? What created the atmosphere on Earth? 4. What is the structure of the atmosphere? 5. What is the function of the atmosphere? 9-11 ES2B Climate is determined by energy transfer from the sun at and near Earth's surface. This energy transfer is influenced by dynamic processes such as cloud cover and Earth's rotation, as well as static conditions such as proximity to mountain ranges and the ocean. Human activities, such as burning of fossil fuels, also affect the global climate. External structures and processes (solar energy): 6. Why are there seasons? 7. How are clouds formed? 8. What causes global climate? (rainforest, desert, plate boundary, basalt layers, glaciers, Puget Sound, erosion, flooding, rain shadow effect) 9. What is the effect of water on the planet? 10. What is heat capacity? 9-11 ES2C Earth is a system that contains essentially a fixed amount of each stable chemical element existing in different chemical forms. Each element on Earth moves among reservoirs in the solid Earth, oceans, atmosphere, and organisms as part of biogeochemical cycles driven by energy from Earth's interior and from the Sun. “How will we know if they have learned it?” (Assessment Tasks--evidence of learning will come from . . . ) Individual summative assessment: Climate and seasons needs energy transfer questions “What will we have them do to learn and practice it?” (Instructional Activities) “What learning and practice materials are needed?” (Materials) Earth Structure Probe External structures and processes (solar radiation and the warming of the Earth): Seasons (solar radiation, terrestrial radiation): Intro to insulation lab—world map plot 3 countries at 0, 30, 60 degrees latitude north and south, assumptions about angle (latitude) and temperature Insulation lab (probe taped to ruler and placed at different angles to light source) demo Flash light on globe to measure angle of sunlight Weather & Climate probe Probe on the difference between weather and climate Climate and Weather (air pressure, air density, air temperature, difference between green house effect (natural process has always occurred) & global warming / change / destabilization (human effect/impact of additional carbon production) ozone layer) Key Terms: transfer of energy, saturation, heating capacity, temperature variation, geologic time, water cycle, glaciation, rain shadow, air pressure, air density, air temperature, difference between green house effect & global warming (change, destabilization), ozone layer, solar radiation, terrestrial radiation Convection in air currents: Coriolis Effect on atmosphere and ocean currents (from earth’s rotation), Oversimplified cells (vertical convection movement motion of air masses rising at equator when heated, rises and moving North or South toward poles, cold air circles and returns underneath it) Ocean’s Impact on Global Climate: Heat Capacity, Graphing coastal vs. inner cities, El Nino and La Nina, 10 Atmospheric Layers and Water Cycle Powerpoint (LINK) Air mass demonstrations with balloons (weigh empty, fill and weigh again), heated aluminum can placed in ice water (crushes) You tube video on the coriolis effect: http://www.youtube.com/watch?v=Wda7azMvabE Biology Unit Sequence: 11 weeks (SHS), 8 weeks (BHS) “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) Mastery of these Biology standards expected for science students. 9th grade lab Ecology (both BHS & LHS): 9-11 LS2E Interrelationships of organisms may generate ecosystems that are stable for hundreds or thousands of years. Biodiversity refers to the different kinds of organisms in specific ecosystems or on the planet as a whole. (BHS will also do 9-11 LS2A, test spec #1-3, and LS2C) Cells (SHS only): 9-11 LS1C Cells contain specialized parts for determining essential functions such as regulation of cellular activities, energy capture and release, formation of proteins, waste disposal, the transfer of information, and movement. Evolution (SHS to start 2012-13): 9-11 LS3A Biological evolution is due to: (1) genetic variability of offspring due to mutations and genetic recombination, (2) the potential for a species to increase its numbers, (3) a finite supply of resources, and (4) natural selection by the environment for those offspring better able to survive and produce offspring. “What questions does the work in this unit answer?” (Unit Guiding Questions) “How will we know if they have learned it?” (Assessment Tasks) “What will we have them do to learn and practice it?” (Instructional Activities) “What learning and practice materials are needed?” (Materials) Ron Thompson Materials: Green = Content ‘Guiding Questions’, Red = Curriculum Resource Guide Book, Black = Section and Pages Numbers from Curriculum Resource Guide Book Bridge from Earth Systems: How does the sun’s energy transfer to living organisms? 3 EOC formatted Field Studies: Pathway Proximity, Rainfall Worm Number, Sunlight Leaf Number Ecology 1. How does the sun’s energy move between living organisms? 2. What is the carbon cycle? Why is it important to living things? 3. What makes something alive? What do all living things have in common? 4. How do we classify the diverse types of life? What is the classification system? 5. What is an ecosystem? What are the levels of organization in an ecosystem? 6. How does the environment impact the organisms in a particular ecosystem? Organization of Life Characteristics of Life (3 days)--What do all living things have in common? Biology: As Scientific Inquiry—Unit One Introduction: 1-3 Characteristics of Life (pp. 5-6) (All living things…Are made of cells, Reproduce as a species, Metabolize, Grow and Develop, Respond to their environment) Classification/Diversity of Life What is the classification system to organize all living things? Classification and Evolution—Unit VII: 39-3 Introduction to Biological Classification (pp. 6-7) DROP??? Ecology Plan--Basic Ecology (4 weeks)--species, populations, communities, ecosystem (levels),Trophic Levels – producers, consumers, food webs, energy, field study) GOULD FOOD WEB web resource (insert link) Abiotic vs Biotic--How is the carbon cycle relevant to living things? Cells and Cell Processes—Unit II: 7-6 (p. 118) What is the difference between a biotic and abiotic environment? Use biotic and abiotic resources from the 10th grade team (content not in the Curriculum Resource Guides)-What are the levels of organization of an ecosystem? (ecosystem → communities → populations → organism) Ecology—Unit VIII: 49-2 Population Dynamics: An Introduction (p. 37) SHS ONLY Macromolecules & Cells 1. What are macromolecules? 2. What are the monomers that make up macromolecules? 3. What are the functions of each macromolecule? 4. What is the function of an enzyme? 5. What is a cell? 6. How does a cell function as a system? 7. What different kinds of cells exist? 8. How do plant and animal cells differ? 9. What are the steps for photosynthesis and respiration? 10. How do sex cells differ from other body cells? 11. What role do adaptations play in species’ survival? Cells (3 weeks) Basic Organelles Identify and label cell parts in diagrams of plant and animal cells. Cells and Cell Processes— Unit II: 4-3 Cell Structure Describe characteristics that distinguish plant cells from animal cells. Complete a cell model. Basic Cell Reproduction (Mitosis/Meiosis) What is the difference between mitosis and meiosis? Reproduction and Development—Unit V: 30-2 Cell Division—Mitosis (pp. 78-80), Connection between cell division and cancer, 30-5 Cell Division—Meiosis (pp. 83-85) What is the purpose of mitosis and meiosis? Same as above. What different kinds of cells exist? Same as above. Macromolecules Identify the monomers that create each macromolecule? Cells and Cell Processes—Unit II: 6-2 Carbohydrates (pp. 86-87), 6-3 Proteins, Fats (pp. 88-90) What is the function of an enzyme? Cells and Cell Processes—Unit II: 9-3 Enzyme Structure and Function Plants vs Animals (*Note: 9th is not addressing prokaryotic vs. eukaryotic cell differences (or domains) Basic Photosynthesis/Respiration 11 What are the steps for the photosynthesis and respiration reactions? Cells and Cell Processes— Unit II: 7-4 Respiration (p. 112), 7-6 Photosynthesis (p. 117) DROP??? Basic Genetics Adaptation What role do adaptations play in species survival (mutations, environment)? Genetics—Unit VI: 32-1 Introduction to Genetics (p. 2) Classification and Evolution—Unit VII: 44-3 Mutation and Natural Selection (pp. 51-52) Basic Evolution What is meant by ‘survival of the fittest’? Classification and Evolution—Unit VII: 44-2 Natural Selection & Evolution ( 49-50) Independent Science Project (BHS Only): 4 weeks “What do we want the students to know or be able to do?” (Power Standard Addressed--could be from State GLEs, National, or Program Certification Standards) “What questions does the work in this unit answer?” (Unit Guiding Questions) INQB Scientific progress requires the use of various methods appropriate for answering different kinds of research questions, a thoughtful plan for gathering data needed to answer the question, and care in collecting, analyzing, and displaying the data. INQC Conclusions must be logical, based on evidence, and consistent with prior established knowledge. 12 “How will we know if they “What will we have them do have learned it?” to learn and practice it?” (Assessment Tasks--evidence (Instructional Activities) of learning will come from . . .) “What learning and practice materials are needed?” (Materials)