1.2.1.A Electron Theory
advertisement

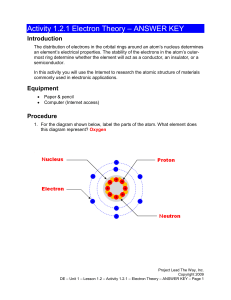
Activity 1.2.1 Electron Theory Introduction The Bohr model of the atom was introduced in 1913. It is an early representation of the atom often introduced in chemistry to describe energies associated with electrons based on their location to the nucleus in the atom. While newer models of the atom are more accurate, the Bohr model was instrumental in understanding how elements are placed on the periodic table, and the properties that an element has based its number of valence shell electrons. The distribution of electrons in the orbital rings around an atom’s nucleus can help determine an element’s electrical properties. The stability of the electrons in the atom’s outer-most ring are one factor that helps determine whether an element will act as a conductor, an insulator, or a semiconductor. It is also important to look at the physical properties of the element when trying to determine which might be a better choice as a conductor, insulator, or semiconductor (state at room temperature, melting point, oxidation-reactivity with other metals). Cost and availability should also be considered. Combinations of elements can create new materials with desired properties that an element alone might not have. In this activity you will use the Internet to research the atomic structure of materials commonly used in electronic applications. http://www.ptable.com/ is a resource that can be used to investigate the properties of some important elements in determining if they are good conductors, insulators, or semiconductors. Equipment Paper & pencil Computer (Internet access) © 2009 Project Lead The Way, Inc. DE Activity 1.2.1 Electron Theory – Page 1 Procedure 1. For the diagram shown below, label the parts of the atom. What element does this diagram represent? Element: ___________oxygen________________ Nucleus proton - - + + + + + + + - - + - - neutron electron 2. Shown below are the electron orbital rings for the elements Lead (Pd) and Tin (Sn). Research or use you knowledge of chemistry to complete the diagrams by labeling the number of electrons that belong in each of the orbital rings in the Bohr model of the atom. 2, 8, 18, 16, 9, 2 Lead (Pb) Electron Bands © 2009 Project Lead The Way, Inc. DE Activity 1.2.1 Electron Theory – Page 2 Tin (Sn) Electron Bands 2, 8, 18, 18, 2 Based on their electron configuration alone, can you determine if tin and lead are good conductors? Conclusion 1. What are the three basic particles that make up all atoms? Proton, electron, neutron 2. What are some of the characteristic of an element that makes it a good conductor? An element with free electrons makes a good conductor. 3. Give three examples of elements that make good conductors. Copper, silver, and gold are three examples of elements that make good conductors. 4. What are some of the characteristic of an element that makes it a good insulator? The number of electrons on the shell are stable makes a good insulator. © 2009 Project Lead The Way, Inc. DE Activity 1.2.1 Electron Theory – Page 3 5. Give three examples of materials that make good insulators. Glass, plastic, and rubber are three examples of materials that make good insulators. 6. Do you remember what is made from tin and lead? Why is this material a good choice for use in electronics? Soldering is made from tin and lead. This is a good choice for electronics because it is a good conductor. © 2009 Project Lead The Way, Inc. DE Activity 1.2.1 Electron Theory – Page 4