Avogadro*s Number
advertisement

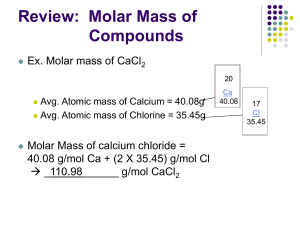
Notes for Avogadro’s Number “The Mole” Power Point 1. Who gave this important number its name? 2. Why can’t atoms and molecules be counted directly? 3. Avogadro’s number is a fundamental physical constant that relates any quantity at the __________ scale to its corresponding ____________________ scale. 4. Loschmidt evaluated the number of molecules in a given ____________________. 5. What is Brownian movement? 6. Millikan was experimenting on a drop of __________ when he revised Avogadro’ number. 7. Another revision occurred as ____________________ counted the alpha particles emitted from radium or uranium. 8. Nuoy’s investigations of molecular monolayers on _______________ refined Avogadro’s number even further. 9. What is the final number attributed to Avogadro’s number? 10. What do we normally count with this number? 11. Explain why it is an excellent number for counting these. 12. Comparing dozens and moles: a. One dozen roses = ___________________ roses. b. ½ dozen roses = ___________________ roses. c. 3.5 dozen roses = ___________________ roses. d. 10 dozen roses = ___________________ roses. e. One mole of roses = ___________________ roses. f. ½ moles of roses = ___________________ roses. g. 3.5 moles of roses = ___________________ roses. h. 10 moles of roses = ___________________ roses. 13. If we want to find the number of roses in 7.5 dozen roses, we set up a conversion factor. A conversion factor is a fraction used to convert between units. Conversion factors always equal one. a. We know one dozen of any substance is 12 of that substance. b. The conversion factor reciprocals are equal to each other 12 items___ 1 dozen items = 1 dozen items 12 items c. If you know how many dozens of roses you have but want to know the quantity: d. 7.5 dozen roses x 12 roses____ = 1 dozen roses e. If you know the quantity of roses and want to find the dozens of roses use the reciprocal: f. 126 roses x 1 dozen roses = 12 roses 14. The mole, commonly abbreviated mol, is the SI unit used to measure the amount of a substance. What does SI stand for? 15. A mole is the number of atoms in exactly 12 grams of a particular isotope of carbon (C-12). If you have 12.00 g of C-12 you have 6.022 x 1023 carbon atoms. a. How many moles of C do you have if you have 6.00 g of carbon? b. How many carbon atoms does 6.00 g of carbon contain? 16. For all elements, a mole is the atomic mass of that element expressed in grams. a. If you have one mole of magnesium, how many Mg atoms do you have? b. If you have 24.31 g of Mg, how many atoms of Mg do you have? c. If you have one mole of Mg, what mass of Mg do you have? 17. One __________ of a compound is 6.02 x 1023 units of that compound (molecules or formula units). 18. The compound magnesium chloride is written MgCl2. a. How many formula units of MgCl2 would one mole of MgCl2 have? 19. We know one mole of any item is 6.02 x 1023 of that item. If I wanted to know how many Mg atoms 7.5 moles of Mg contained, we can set up a conversion factor using this information. a. 7.5 moles Mg x 6.02 x 1023 atoms = 1 mole Mg b. If you know the quantity of atoms and want to find the quantity of moles: c. 2.40 x 1018 Mg atoms x 1 mol Mg = 23 6.02 x 10 Mg atoms 20. What is iron’s (Fe) atomic mass? 21. If you had 167.55 g of Fe, how many moles of Fe would you have? a. Create a conversion factor to help you solve this problem. 22. If you had 8. 0 g of O, how many moles of oxygen would you possess? 23. If you measured out 9.64 mol O, how many grams of oxygen would that be? a. Create a conversion factor to help you solve this problem.