CHEM 442 Lecture 22 Problems 22
advertisement

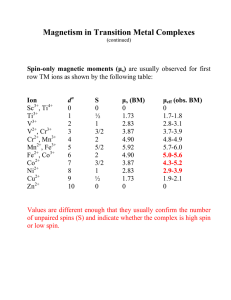
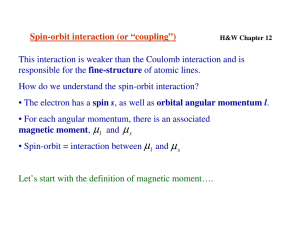
CHEM 442 Lecture 22 Problems 22-1. The neon atom has the configuration (1s)2(2s)2(2p)6. Let the operator for the sum of 10 all z-component orbital angular momentum be å lˆ ( r ) , where r z n n is the spatial n=1 coordinates of the nth electron. Let the operator for the sum of all z-component spin 10 angular momentum be å ŝ (s ) , where s z n n is the spin coordinates of the nth electron. n=1 Show that the total z-component orbital and spin angular momenta of the neon atom are zero. Argue that the total orbital and spin angular momenta of the neon atom are also zero. 22-2. Identify the levels that may arise from the configurations and their degrees of degeneracy: (a) (3s)1, (b) (3p)1. 22-3. Identify the levels that may arise from the configurations and their degrees of degeneracy: (a) (4d)1, (b) (4f)1. 22-4. Suggest the first-order perturbation expression for the energy shift caused by the spin-orbit coupling. Use the spin-orbit coupling constant A given in units of cm−1. ( ) 1 2 ˆ2 2 22-5. Show that ŝ × lˆ = ĵ - l - ŝ and evaluate the expression of 22-4. 2 22-6. Using the result of 22-5, evaluate the spin-orbit energy shifts in (a) (3s)1, (b) (3p)1. 22-7. The potassium emission spectra includes two lines at 766.49 nm and 769.90 nm. Determine the spin-orbit coupling constant A for the 4p orbital of K. 22-8. Find the lithium emission lines corresponding to the (1s ) ( 2 p ) ® (1s ) ( 2s ) from the NIST Atomic Spectroscopy Database (http://www.nist.gov/pml/data/asd.cfm). Determine the spin-orbit coupling constant A for the 2p orbital of Li. 2 1 2 1 22-9. What are the differences between fluorescence and phosphorescence? Why is phosphorescence more common in heavy-element compounds? 22-10. What is an intersystem crossing?