Hydrofluoric acid solutions - School of Materials Intranet
advertisement

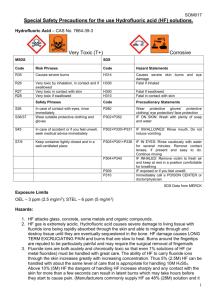
SOM017 Special Safety Precautions for the use Hydrofluoric acid solutions. Hydrofluoric Acid – CAS No. 7664-39-3 Very Toxic (T+) MSDS Corrosive SDS Code Risk Phrases Code Hazard Statements R35 Causes severe burns H314 R26 Very toxic by inhalation, in contact and if swallowed Very toxic in contact with skin Very toxic if swallowed H330 Causes severe skin burns and eye damage Fatal if inhaled H300 H310 Fatal if swallowed Fatal in contact with skin Safety Phrases Code Precautionary Statements In case of contact with eyes, rinse immediately Wear suitable protective clothing and gloves P280 Wear protective gloves/ protective clothing/ eye protection/ face protection IF ON SKIN: Wash with plenty of soap and water S45 In case of accident or if you feel unwell, seek medical advice immediately P302+P330+P331 IF SWALLOWED: Rinse mouth. Do not induce vomiting S7/9 Keep container tightly closed and in a well-ventilated place P305+P351+P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continue rinsing IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing IF exposed or if you feel unwell: Immediately call a POISON CENTER or doctor/physician R27 R28 S26 S36/37 P302+P352 P304+P340 P309 P310 SDS Data from MERCK Exposure Limits OEL – 3 ppm (2.5 mg/m3); STEL – 6 ppm (5 mg/m3) Hazards: 1. HF attacks glass, concrete, some metals and organic compounds. 2. HF gas is extremely acidic. Hydrofluoric acid causes severe damage to living tissue with fluoride ions being rapidly absorbed through the skin and able to migrate through and destroy tissue until they are eventually sequestered in the bone. HF damage causes LONG TERM EXCRUCIATING PAIN and burns that are slow to heal. Burns around the fingertips are reputed to be particularly painful and may require the surgical removal of fingernails 3. Fluoride ions are both acutely and chronically toxic so that even 1% solutions of HF (or metal fluorides) must be handled with great care. The ability of HF to carry fluoride ions through the skin increases greatly with increasing concentration. Thus 5% (2.5M) HF can be handled with about the same level of care that is appropriate for handling 10M H 2S04. Above 10% (5M) HF the dangers of handling HF increase sharply and any contact with the skin for more than a few seconds can result in latent burns which may take hours before 1 SOM017 they start to cause pain. (Manufacturers commonly supply HF as 48% (28M) solution and it may be supplied as strong as 73% (44M). Handling HF of these concentrations is FAR MORE DANGEROUS than handling other common concentrated acids.) 4. Exposure to concentrations, above 50% will cause immediate pain, between 20% and 50% will cause pain up to 8 hours later, less than 20% may cause pain up to 24 hours later. Storage: 1. Only ever order just enough to cover requirements. 2. Hydrofluoric acid should only be stored in a screw capped polyethylene (or equivalent) container in a cool well-ventilated area. Its presence should be clearly indicated. 3. The School safety advisor must be contacted before any research involving HF gas commences. 4. All containers must be clearly labelled with content, warning signs and who the solution belongs to. 5. When not in use ALL chemical solutions must be stored in a chemical cabinet. Precautions and Procedures: 1. NO WORK INVOLVING HF CAN BE CARRIED OUT OUTSIDE NORMAL WORKING HOURS. 2. Do you have to use hydrofluoric acid? – No! Then don’t. a. Is there safer alternative? – If so use it. b. What is the lowest concentration to achieve task? – use it. c. What is the smallest volume necessary to achieve task? – use it. 3. Prior to using hydrofluoric acid a Risk and COSHH assessment for the dilution of commercially supplied HF to the correct concentration should be carried out as well as an assessment for the disposal of any surplus acid. All HF solutions must be properly labelled. 4. All persons who wish to use hydrofluoric acid must see the staff member responsible for the area where the work is to be carried out for training in HF awareness and procedures. 5. Complete a separate COSHH assessment for the actual experiment. 6. Reactions involving HF gas can not be carried out. 7. Reactions involving hydrofluoric acid can only be carried out in designated labs in fume cupboards with a high extraction rate and good containment. HF warning notices should be on display, on the laboratory door and where the experiment is being carried out. 8. Ideally have a gentle flow of water around the base of container of HF solution to dilute any small spillages or drips. 9. Appropriate safety equipment should be used i.e. safety goggles, or when appropriate a face shield (i.e. with large volumes), clean good quality lab coat and when appropriate chemically resistant apron or suit and HF resistant gloves. The gloves should be checked regularly and carefully for any damage especially pin-holes (this might be done by filling them with water, before and after use, drying before use or putting them away ready for use again). 10. Ideally, each HF user should have there own set of HF protective gloves. 2 SOM017 11. Wash gloved hands regularly even when using dilute hydrofluoric acid. 12. The researcher must check before each session of experimental work that the emergency pack of calcium gluconate gel (see below) is present in the first aid box within the laboratory and be aware of the location of the nearest phone and first aider and sufficient 3M NaHCO 3 solution neutraliser is available. 13. Always wash hands before leaving work area. 14. All HF solutions must be stored in a locked metal chemical cabinet which is only used for HF solutions. All HF solutions required for further work must be returned to the HF cabinet immediately after use. 15. All HF protective equipment, fume cupboard and all equipment used with HF must be made safe after experimental work is complete and stored in the appropriate cupboard. The metal front sill of the fume cupboard must be left clean and dry. THIS MEANS CLEAN UP! 16. If using large volumes, these safety measures must be followed in addition to all procedures above: a. Use of large volumes can only be carried out in designated areas and must have a full body drench shower. b. Try to keep to volumes to less than 1000 ml. c. Use of a containment spill tray of at least 5 times the volume of acid must be used. d. Sufficient quantity of 3M NaHCO3 solution neutraliser must be available. e. Full front protective apron or suit must be worn. Protective footwear should be worn for larger volumes. f. Ideally the spill tray should have a continuous flow of water, to dilute and wash away any spillages (see diagram 1). Keep contamination outside the tray to a minimum. g. To minimise the hazard when decanting HF solutions to and from the storage container and working container use plastic funnel and/or smaller plastic beaker. 17. All suspicious wet areas should be tested with litmus paper and dealt with accordingly using sodium bi-carbonate solution. Disposal of Hydrofluoric acid solutions 1. Small volumes of low concentrations, i.e. less than 100 ml and 5% HF, should first be diluted slowly and in small amounts to a large volume of water then with copious amounts of water down the drain, i.e. with running water for at least 15 minutes ( more than 50 volumes). Only hydrofluoric acid solutions not contaminated with metals may be disposed down the sewer. Solutions contaminated with metals must be stored and appropriately disposed of via a waste management company. 2. Large volumes, greater than 100ml, must be stored in waste HF acid bottles that are no larger than 2.5 litres and properly labelled. Decanting of work solution into the waste HF acid bottle must be done within the fume cupboard, following all the guidelines contained in this document. The waste HF acid bottle must be kept in the HF acid cabinet when not in use. When the waste HF acid bottle is nearly full it is to be placed in the outside chemical store and the stores person requested to have it disposed of. 3. Large volumes with concentrations greater than 5% HF must be carefully diluted to below 5% before adding to the waste HF acid bottle. 3 SOM017 In the event of an accident: 1. Remove all contaminated clothing and flood the affected areas with large volumes of water for at least 10 minutes. 2. A first aider should be summoned. The 2% calcium gluconate gel should be applied to the affected area using neoprene gloves and continuously massaged into the skin for at least 15 minutes. Cover the area with a dressing soaked in the gel and lightly bandage. 3. When giving first aid, protect yourself and the casualty from further exposure. 4. Splashes to the eyes are particularly painful and dangerous and treated by flooding with water for at least 15 minutes. 5. To send casualty to Accident and Emergency department or local Eye casualty department. To summon an ambulance to transport the casualty to hospital dial 9-999 on any internal phone or ring 69966 or 0161-306-9966 to contact security. The above first aid procedures can be continued during transit. 6. Any contaminated clothes or gloves must be double bagged and labelled for later disposal. 7. If left untreated HF burns can result in extensive and permanent damage, which may extend to the underlying bone. Diagram 1 Water in Water out – directly to drain Wash tank HF solution 4