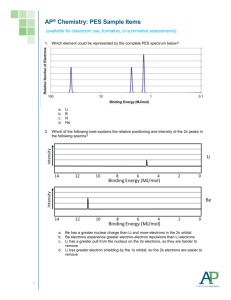
Chapter 2 Review Questions – Atomic Structure and Theory
advertisement
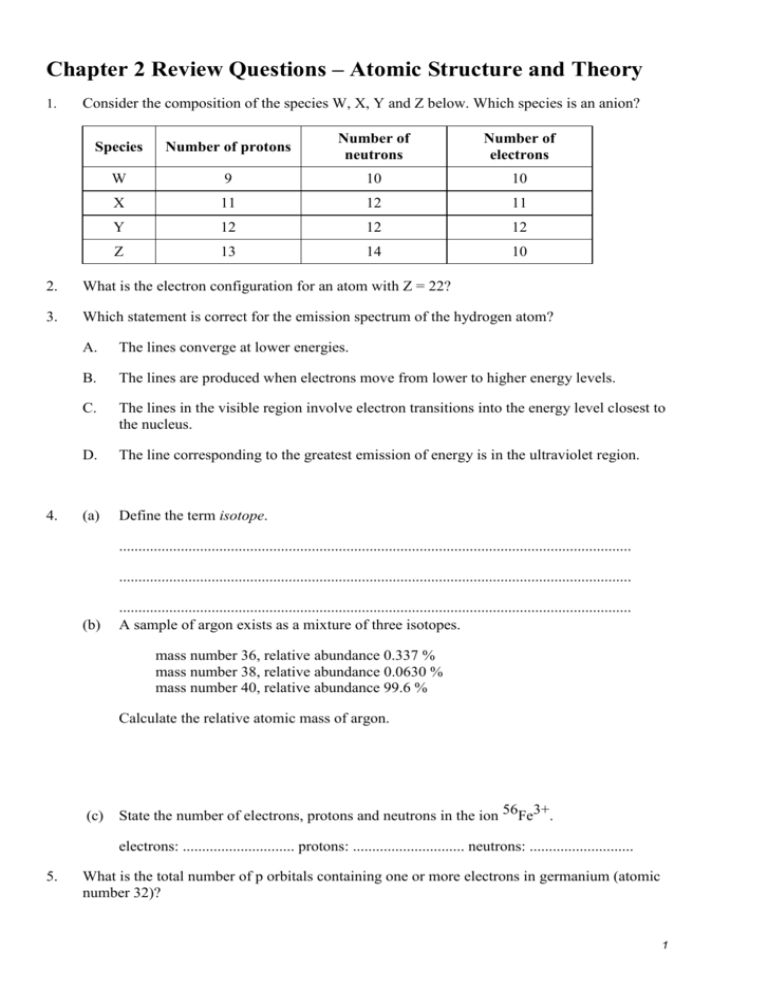
Chapter 2 Review Questions – Atomic Structure and Theory 1. Consider the composition of the species W, X, Y and Z below. Which species is an anion? Species Number of protons Number of neutrons Number of electrons W 9 10 10 X 11 12 11 Y 12 12 12 Z 13 14 10 2. What is the electron configuration for an atom with Z = 22? 3. Which statement is correct for the emission spectrum of the hydrogen atom? 4. A. The lines converge at lower energies. B. The lines are produced when electrons move from lower to higher energy levels. C. The lines in the visible region involve electron transitions into the energy level closest to the nucleus. D. The line corresponding to the greatest emission of energy is in the ultraviolet region. (a) Define the term isotope. ..................................................................................................................................... ..................................................................................................................................... (b) ..................................................................................................................................... A sample of argon exists as a mixture of three isotopes. mass number 36, relative abundance 0.337 % mass number 38, relative abundance 0.0630 % mass number 40, relative abundance 99.6 % Calculate the relative atomic mass of argon. (c) State the number of electrons, protons and neutrons in the ion 56Fe3+. electrons: ............................. protons: ............................. neutrons: ........................... 5. What is the total number of p orbitals containing one or more electrons in germanium (atomic number 32)? 1 6. (i) State the full electron configuration for argon. …………………………………………………………………………………………… (ii) 7. Give the formulas of two oppositely charged ions which have the same electron configuration as argon. …………………………………………………………………………………………… What are the two differences and two similarities between two neutral atoms represented by the symbols 59 27 Co and 59 28 Ni ? 8. The electron arrangement of sodium is 2.8.1. How many occupied main electron energy levels are there in an atom of sodium? 9. State the complete electronic configuration of bromide ion, Br1- and the iron(III) ion, Fe3+. Br1-: …………………………………………………………………………………. Fe3+: …………………………………………………………………………………. 10. What is the wavelength of light, in nanometers, whose energy is 8.88 x 10 -19 J? In what region of the electromagnetic spectrum would this wavelength be found? 11. Calculate the wavelength of the radiation when an electron moves from n = 1 to n =5. Is this line in the visible region of the electromagnetic spectrum? Is the movement of this electron an exothermic or an endothermic process? 12. What are two similarities and two differences between the electrons of the 3d sublevel and the electrons of the 3p sublevel? 13. Given the principal quantum number n = 5, what are all of the possible values for l and ml? What is the maximum number of electrons that could be held in the n = 5 energy level? 14. Explain why the following set of quantum numbers is not possible: n = 4, l = 4, ml = ½ 2 15. Determine the energy level and sublevel (1s for example) that an electron would occupy if it had the following set of quantum numbers: n = 3, l =1. How many total electrons could fit this description of quantum numbers? 16. Write out the electron configuration for Radium (Ra). 17. Write the condensed or noble gas configuration for Hafnium (Hf). 18. Draw the orbital notation for Vanadium. Make sure to include all of the orbitals and label each of them. 19. What are the steps, in the correct order for using a mass spectrometer? Briefly describe what happens during each of these steps. What does the graph of the detection analysis tell us about the injected sample? 20. In which of the following elements (Al, Na, Mg, Si) will there be an extremely large jump between the 1st ionization energy and the 2nd ionization? Explain your answer. 3