SPS2: Classifications of matter (SPS2classificatonofmatter)
advertisement
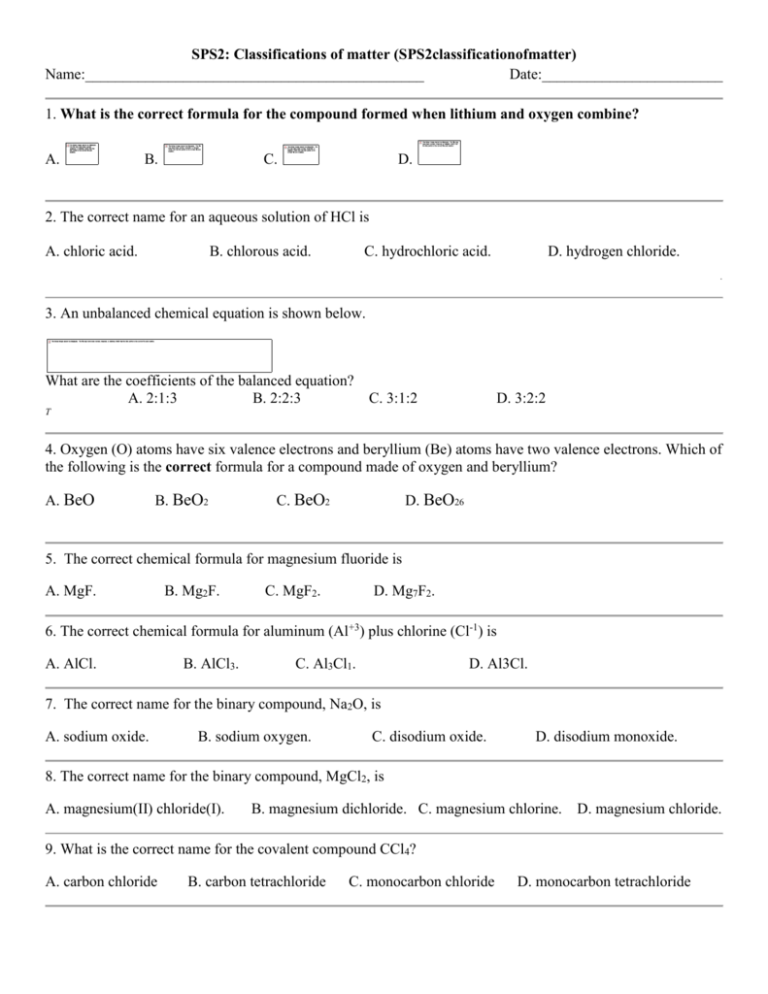
SPS2: Classifications of matter (SPS2classificationofmatter) Name:_____________________________________________ Date:________________________ 1. What is the correct formula for the compound formed when lithium and oxygen combine? A. B. C. D. 2. The correct name for an aqueous solution of HCl is A. chloric acid. B. chlorous acid. C. hydrochloric acid. D. hydrogen chloride. . 3. An unbalanced chemical equation is shown below. What are the coefficients of the balanced equation? A. 2:1:3 B. 2:2:3 C. 3:1:2 D. 3:2:2 T 4. Oxygen (O) atoms have six valence electrons and beryllium (Be) atoms have two valence electrons. Which of the following is the correct formula for a compound made of oxygen and beryllium? A. BeO B. BeO2 C. BeO2 D. BeO26 5. The correct chemical formula for magnesium fluoride is A. MgF. B. Mg2F. C. MgF2. D. Mg7F2. 6. The correct chemical formula for aluminum (Al+3) plus chlorine (Cl-1) is A. AlCl. B. AlCl3. C. Al3Cl1. D. Al3Cl. 7. The correct name for the binary compound, Na2O, is A. sodium oxide. B. sodium oxygen. C. disodium oxide. D. disodium monoxide. 8. The correct name for the binary compound, MgCl2, is A. magnesium(II) chloride(I). B. magnesium dichloride. C. magnesium chlorine. D. magnesium chloride. 9. What is the correct name for the covalent compound CCl4? A. carbon chloride B. carbon tetrachloride C. monocarbon chloride D. monocarbon tetrachloride 10. During science lab, Mr. Smith's students tested the reactivity of various metals in sulfuric acid. When zinc was added to sulfuric acid, bubbles were produced, a sure sign that a chemical reaction had taken place. Based on the law of conservation of matter, if 30 grams of zinc are added to 45 grams of sulfuric acid to produce just 1 gram of hydrogen gas, how much zinc sulfate would also be produced? The formula for the chemical reaction is as follows: Zn + H2SO4 ZnSO4 + H2 A. 15 grams B. 30 grams C. 45 grams D. 74 grams 11. Which of these equations correctly represents a balanced synthesis reaction? A. Na + Cl NaCl B. 2Na + Cl2 2NaCl C. NaOH + HCl NaCl + HOH D. 2Na + O Na2O 12. Hydrogen peroxide decomposes to form water and oxygen. This reaction is represented by which of these balanced equations? A. H2O2 H2O + 2O B. H2O + O2 H2O2 C. 2H2O2 2H2O + O2 D. 2H2O2 2H2O + 2O 13. In Antoine Lavoisier's classic experiment, mercuric (II) oxide is heated in a sealed container. The solid red powder is changed into silver liquid mercury and oxygen gas. If Lavoisier heated 50 grams of powdered mercuric oxide to produce 46.5 grams of liquid mercury, how much oxygen would be released? A. 3.5 grams B. 16 grams C. 32 grams D. 96.5 grams 14. Name the following compound: N2O5 A. nitrous oxide B. nitrogen oxide C. nitrogen pentoxide D. dinitrogen pentoxide 15. Aluminum oxide decomposes to produce aluminum plus oxygen gas. Identify the balanced equation for this decomposition reaction. A. Al2O3 2Al + 3O B. Al2O3 2Al + 3O2 C. 2Al2O3 4Al + 3O2 D. 4Al + 3O2 2Al2O3 16. The coefficients of the correctly balanced equation for the reaction illustrated above are — A. 1, 1, 1. B. 1, 1, 2. C. 2, 1, 2. D. 2, 2, 1. P 17. The formula for lithium nitride is — A. B. C. D. 19. When is heated in a crucible, there is a loss of water. How should a student determine the amount of water lost? A. Subtract the mass of the from the mass of B. Subtract the mass of the from the mass of C. Add the masses of and D. Multiply the masses of and P 20. A balanced chemical equation has equal numbers of atoms of each type on both sides of the equation. This illustrates the principle of — A. conservation of energy C. action and reaction B. conservation of mass D. natural selection 21. The appropriate model for a decomposition reaction is — A. B. C. D. 22. The formula for dinitrogen tetroxide is — A. B. C. 23. hydrochloric acid will be — A. 2. B. 3. D. When the above equation is balanced, the coefficient of the C. 4. D. 6. — B. phosphorus oxide. C. phosphorus (II) oxide. D. diphosphorus pentoxide. 24. The correct name for A. phosphorus (V) pentoxide. 25. Which type of reaction is represented here? A. Single replacement B. Double replacement C. Synthesis D. Decomposition 26. The correct formula for copper (I) bromide is — A. B. C. D. Answer Key 16. D) 2, 2, 1. 1. B) 2. C) hydrochloric acid. 3. A) 2:1:3 17. B) 18. A) Decomposition 4. A) BeO 19. A) Subtract the mass of the 5. C) MgF2. mass of 6. B) AlCl3. 20. B) conservation of mass 7. A) sodium oxide. 8. D) magnesium chloride. 9. B) carbon tetrachloride 21. A) 10. D) 74 grams 11. B) 2Na + Cl2 12. C) 2H2O2 2NaCl 2H2O + O2 23. D) 6. 24. D) diphosphorus pentoxide. 13. A) 3.5 grams 14. D) dinitrogen pentoxide 15. C) 2Al2O3 22. A) 4Al + 3O2 25. B) Double replacement 26. A) from the









