Ocean Salinity & Density Currents Class Copy
advertisement

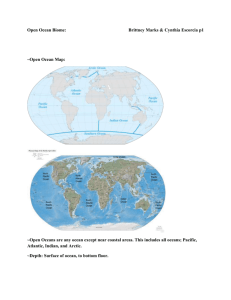
Ocean Salinity & Density Currents Class Copy Ocean Salinity Oceans are salty. The rain that fell to Earth’s surface billions of years ago washed over rocks and dissolved minerals. The minerals contained substances that formed salts. Rivers and streams carried these substances to ocean basins. Underwater volcanoes also contributed substances that could form salts. Salts make up 99.7% of the ocean’s dissolved materials. The common term to describe the dissolved salts in seawater is salinity. Ocean water contains many different kinds of salts, but the most abundant is sodium chloride (NaCl), also known as table salt. Do #1 on worksheet Ocean Salinity Variations Salinity levels (the amount of dissolved salts) vary from one part of the ocean to another. Any variation in salinity levels is influenced by factors that increase or decrease the supply of fresh water into the ocean. Fresh water enters the ocean by precipitation (rain or snow), by runoff from steams and rivers, and by glacial melting. For example, as rivers enter the ocean they carry large volumes of fresh water and decrease the salinity. Fresh water leaves the ocean by evaporation and by formation of ice. Evaporation in hot, dry climates causes an increase in the salinity level. The Dead Sea , located in Israel, has a salinity level 7x greater than that of most ocean water. At the polar regions, the freezing of ocean water increases the salinity of the surrounding water. Do #2, #3 and #4 (on worksheet) Ocean's Vertical Structure Except at the polar regions (high latitudes near 60 degrees to the poles), the ocean is divided into three vertical layers based on density: the mixed (surface) layer, the transition layer (pycnocline), and the deep layer. In the polar regions, the transition layer and mixed layer are absent. This figure shows a crosssectional view of the Atlantic Ocean. The X-axis spans from 60 degrees N (Arctic circle) to 60 degrees S (near Antarctica). The Yaxis shows the depth of ocean. Notice where the mixed layer, the transition layer, and the deep layer are located. Although this figure doesn’t show it, the deep layer extends to the ocean floor (4000 -6000 m). Do #5 on worksheet How does the ocean become layered? The density of ocean water is influenced by the temperature of the water and by the salinity of the water. Saltwater is more dense than freshwater. Very salty water is more dense than less salty water. Cold water is denser than warm water. Cold salty water is much more dense than warm, less salty water. Do #6 on worksheet Mixed or Surface Layer Stirring of surface waters by the wind produces a well-mixed layer of uniform or nearly uniform density. For this reason, the ocean surface is called the mixed layer. Surface currents (mixed layer currents) are changeable, continually responding to variations in the wind, precipitation, and heating or cooling. The wind-driven surface currents are generally among the strongest currents in the ocean. Transition Layer (pycnocline layer) The transition layer, situated between the mixed layer and the deep layer, is where water density increases rapidly with depth because of changes in temperature and/or salinity. Recall that cold water is denser than warm water and salty water is denser than fresh water. Deep Layer The dark, cold deep layer below the transition layer accounts for most of the ocean's mass. Within the deep layer, density continues to increase gradually with depth. Water moves slowly and doesn’t mix much; in only a few locations (usually near the bottom) are water movements fast enough to be considered currents. Do #7 on worksheet How ocean layers form The ocean's three-layer structure is an example of how gravity separates a fluid into layers such that the density of each layer is less than the density of the layer below it. More dense fluids sink and less dense fluids rise. The ocean's transition layer is very stable thus minimizing any mixing between the mixed layer and deep layer; Stability (stable state of equilibrium) refers to vertical motions of ocean water. A system is described as stable if it tends to persist in its original state without changing. Following a disturbance (i.e., a storm), a stable system returns to its initial state or condition. As noted above, the usual stable state of the ocean has a layer of water that is warmest near the surface (the mixed layer) Underneath the surface layer is the transition layer that becomes denser with increasing depth. Strong storm winds may temporarily disturb this stable (layering) stratification, bringing colder than usual water to the surface. Once the wind slackens, however, the original layered structure is soon restored. Do #8 on worksheet Freezing of Ocean Water Fresh water freezes and ice melts at 0°C (32°F). 0°C is the melting point of ice and the freezing point of water. The number of molecules of solid water melting equals the amount of liquid water molecules freezing. When the rate of freezing is the same as the rate of melting, the amount of ice and the amount of water won't change. The ice and water are said to be in a dynamic state of equilibrium with each other, therefore there is no net change in either quantity. Do #9 on worksheet However, freezing occurs at a greater rate than melting when the water temperature drops below 0°C. Because there are more water molecules being captured by the ice (being frozen) than there are ice molecules turning to liquid, the net result is that the amount of ice increases. Sea ice changes the salinity of the ocean Although sea ice is made from salty seawater, the salt molecules are kept out of the ice as it forms. This is because as water freezes the molecules are arranged in a precise and rigid way, a lattice. There isn't much room for salt molecules to be trapped in the lattice-like structure of ice. Because the salt is kept out of frozen water, the salinity increases in the surrounding oceans. Do #10 on worksheet The Global Conveyor Belt Currents circulate the globe. Some are wind-driven surface currents. Some currents are caused by differences in water temperature and salinity from one area to another. These latter currents are part of the global conveyor belt. Global climate change could disrupt the global conveyer belt, causing potentially drastic temperature changes in Europe and even worldwide. A. The conveyor belt begins on the surface of the ocean near the pole in the North Atlantic. Here, the water is chilled by arctic temperatures. It also gets saltier because when sea ice forms, the salt does not freeze and is left behind in the surrounding water. The cold water is now more dense, due to the added salts, and sinks toward the ocean bottom. Surface water moves in to replace the sinking water, thus creating a current. A. Cold, salty, dense water sinks at the Earth's northern polar region and heads south along the western Atlantic basin B. This deep water moves south, between the continents, past the equator, and down to the ends of Africa and South America. The current travels around the edge of Antarctica, where the water cools and sinks again, as it does in the North Atlantic. Thus, the conveyor belt gets "recharged." B. The current is "recharged" as it travels along the coast of Antarctica and picks up more cold, salty, dense water. Do # 11 on worksheet C. As it moves around Antarctica, two sections split off the conveyor and turn northward. One section moves into the Indian Ocean, the other into the Pacific Ocean. These two sections that split off warm up and become less dense as they travel northward toward the equator, so that they rise to the surface (upwelling). C. The main current splits into two sections, one traveling northward into the Indian Ocean, while the other heads up into the western Pacific. D. They then loop back southward and westward to the South Atlantic, eventually returning to the North Atlantic, where the cycle begins again. D. The two branches of the current warm and rise as they travel northward, then loop back around southward and westward. E. The now-warmed surface waters continue circulating around the globe. They eventually return to the North Atlantic where the cycle begins again. Do #12 on worksheet How much water moves because of the Global Conveyor Belt? The conveyor belt moves at much slower speeds (a few centimeters per second) than wind-driven or tidal currents (tens to hundreds of centimeters per second). It is estimated that any given cubic meter of water takes about 1,000 years to complete the journey along the global conveyor belt. In addition, the conveyor moves an immense volume of water—more than 100 times the flow of the Amazon River . Do #13 on worksheet Climate and the Global Conveyor Belt The global conveyor belt is a strong, but easily disrupted process. If global warming results in increased rainfall in the North Atlantic, and the melting of glaciers and sea ice, the influx of warm freshwater onto the sea surface could block the formation of sea ice, disrupting the sinking of cold, salty water. This sequence of events could slow or even stop the conveyor belt, which could result in potentially drastic temperature changes in Europe. Phytoplankton and the Global Conveyor Belt The conveyor belt is also an extremely important component of the global ocean nutrient cycle and carbon dioxide cycle. Warm surface waters have their nutrients and carbon dioxide used up by microscopic plants, phytoplankton. These waters are “recharged” (enriched again) as they travel through the conveyor belt’s deep or bottom layers. The world’s food chain depends on the cool, nutrient-rich waters that support the growth of algae and seaweed. Do #14 on worksheet Adapted from DataStreme Ocean American Meteorological Society. Mark Schwartz. Stanford Report, April 24, 2002 NASA (National Aeronautics and Space Administration) 2000 Case Study from NOAA (National Oceanic and Atmospheric Administration) This reading highlights the how on oceans interact globally! Sea ice forms, grows, and melts in the ocean. It forms in the polar oceans, the Arctic the Antarctic Oceans. In the Arctic, there is a permanent mass of ice covering the region. Nearly all of the Southern Ocean or Antarctic sea ice is "seasonal ice," or it melts away and reforms annually. Arctic and Antarctic ice is of vital importance to the marine mammals and birds for which it is a habitat. Even though sea ice occurs primarily in the polar regions, it influences the climate of the entire globe. Sea ice is important because it regulates exchanges of heat, moisture and salinity in the polar oceans. It also can influence the success of an ecosystem. In March 2000 (the summer season in the Antarctic), the largest iceberg ever recorded broke off Antarctica's Ross Ice Shelf. Roughly the size of Connecticut, the massive berg eventually fractured into smaller pieces which blocked the normal flow of pack ice out of the Ross Sea. As a result, large stretches of normally open ocean were covered with pack ice from November 2000 to March 2001. During these crucial spring and summer months, the Ross Sea usually teems with life, as tons of microscopic marine algae -- called phytoplankton -- undergo reproductive blooms. Phytoplankton need open water to reproduce, but the abnormally high levels of pack ice caused a 40 percent decline in plankton productivity. Sea ice is very effective at blocking light, so the phytoplankton couldn't grow -- there was just too much ice around. The Ross Sea ecosystem depends on phytoplankton -- a primary food source for shrimp-like krill, which in turn are consumed by fish, seals, whales and penguins. The 40 percent drop in phytoplankton, affected the entire food chain -- from krill to penguins. Penguins suffered breeding losses because of the lack of food; they had to go much farther to feed and their nests were exposed for longer periods of time to predators.