Swarming Motility Assay Protocol
advertisement

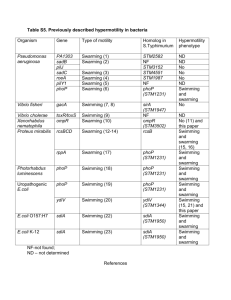
Title: Write your title here A Protocol for Assaying Swarming Motility in Microbes Yangtsho Gyaltshen1 and Brooke Jude1 List authors by first name (optional middle initial or middle name) followed by last name. Separate multiple authors by commas. Use superscript numbers to link authors to specific affiliations, and symbols *,†,†† for author notes (such as corresponding author). For example, First Middle Last1*, First Last2†, and First M. Last1 Affiliations: 1 Bard College: Biology Program 1 Precede each affiliation by a superscript number corresponding to the author list; end each affiliation with a period. 2 Each affiliation should be in a separate paragraph. *Correspondence to: the corresponding author(s) should be indicated with an asterisk; include the postal mail and email addresses of the corresponding author(s). †Additional author notes should be indicated with symbols (for example, for current addresses, type of contribution, etc.). Type of Manuscript: CourseSource Teaching Tools and Strategies Manuscript Funding & Conflict of Interest Statement: Bard College: Biology Program List of Tables, Figures and Supplemental Material: Figure 1. Model of swarming assay protocol and swarming motility over time Title and Description of Primary Image: We ask that an image be submitted with the manuscript that represents the information in the article (e.g. a picture of a dividing cell for a Lesson about mitosis or a picture taken of students doing the activity). This image will be displayed with the title of your article on the CourseSource website. If you do not have a primary image, the editorial staff will select one that best fits your article. Please be conscientious of the copyright associated with any image used in your article. Abstract Page The abstract should be a single paragraph of 250 words or less. Start with an opening sentence that sets the teaching challenge that you address in this manuscript, provide background information and briefly describe your take-home message. This swarming motility assay protocol tool is aimed at enhancing a visual understanding of morphological variability among bacterial strains of environmental isolates. The assay is a variation on traditional Swarming motility protocols modified by a student to characterize motility differences in the transposon mutant strains of iodabacter sp. This serves as a beneficial tool as it can be harnessed as a visual model for genetic for analysis of a gene and subsequent protein function. The simplicity and low-costs associated with this technique make its implementation in variable biology classrooms easily applicable. In addition to simplicity, various media bases can be utilized by reduction of agar concentrations to optimize growth conditions for the microbe of interest. This visual nature of this tool encourages students to apply genetic characterization of microbial to phenotypic variability under different growth conditions. Teaching Tools and Strategies Context Page: To make the submission process easier, you may want to fill out the following form, (you will be asked to select answers during the submission process). Choose all applicable options that effectively describe the Teaching Tools and Strategies. **Please delete this page prior to submission. **Not all categories will pertain to your article, in those cases, please select ‘N/A’ when submitting on the website. · o o o o o Course Biochemistry Cell Biology Genetics Microbiology Molecular Biology · o Course Level Upper Level · o o Class Type Lecture Lab · o o o Audience Life Sciences Major 4-year College University · Class Size o o 1 – 50 51 – 100 · o Lesson Length Multiple class periods · o o o o o o o o o o Key Scientific Process Skills Reading research papers Reviewing prior research Asking a question Formulating hypotheses Designing/conducting experiments Predicting outcomes Gathering data/making observations Analyzing data Interpreting results/data Communicating results · o o o o o o o o o o o o o Pedagogical Approaches Think-Pair-Share Brainstorming Case Study Collaborative Work One Minute Paper Reflective Writing Concept Maps Strip Sequence Computer Model Physical Model Interactive Lecture Pre/Post Questions Other · o o o Bloom’s Cognitive Level (based on learning objectives & assessments) Foundational: factual knowledge & comprehension Application & Analysis Synthesis/Evaluation/Creation · o o Principles of how people learn Motivates student to learn material Focuses student on the material to be learned o o o Develops supportive community of learners Reveals prior knowledge Requires student to do the bulk of the work · o o o o Vision and Change Core Concepts Structure and Function Information flow, exchange and storage Pathways and transformations of energy and matter Systems · o o Vision and Change Core Competencies Ability to apply the process of science Ability to use quantitative reasoning · Key Concepts: List 3 – 10 key concepts (topics) that are relevant for the Teaching Tools and Strategies (e.g. plating; PowerPoint; Large Class; Writing; etc.) o Plating o Pipetting o Basic photography Main Text Begin the Teaching Tools and Strategies text on a new page. 1. Main Text: Describe how to use the resource, tool or approach in as brief and practical way as possible, citing references and related materials. This tool was introduced as a method to assay morphological differences in Iodobacter sp. mutants created in a laboratory setting (Micro module). This is a qualitative visual method to characterize this environmental isolate in respect to its role in bioaugmentation against the fungal pathogen, Batrachochytrium dendrobatidis. This assay was utilized to observe morphological differences in transposon mutants of the Iodobacter sp. This method allowed us to characterize the mutants and can be harnessed as a qualitative tool in genetic analysis of a gene and subsequent protein function. Swarming motility is an organized surface translocation has been observed in both Gramnegative and Gram-positive species and requires the sensing and integration of a variety of environmental, as well as intracellular, signals involving surface contact and local high population density. This serves as an important tool in understanding both cellular motility as well as cell-to-cell interactions. This simple method utilizes a basic understanding of plating and pipetting techniques and can be implemented with a variety of media bases. All materials used by this tool are available in a college laboratory in addition to basic photographic hardware for imaging purposes. Overnight cultures of your organisms of interest are prepared in a liquid media of choice. They could include, but are not limited to 1% tryptone, R2A, and LB liquid media. Your isolates are inoculated into the liquid media and placed on a rotator overnight at room temperature (for iodobacter sp.). The following day, you would carefully pipette 3 uL of the culture onto the center of your swarming plates (eg. 1% Tryptone, R2A and LB) consisting of 0.5% agar (5g/L), allowing the drop to air dry before turning over the plates and storing them at optimum growth temperature. A caveat to keep in mind is the possibility of plate contamination during air drying. For the best results, air drying should be done under a sterile hood and the lids should be replaced as soon as possible. Multiple isolates can be observed on the same plate to observe swarming interactions. These inverted plates are incubated at the organism's ideal temperature for 24 to 48 hours before making swarming observations. Swarming motility and growth patterns are observed and photographed using a flatbed scanner daily over the course of ten to fourteen days (depending on speed of growth) The laboratory techniques used can be implemented at even an introductory course level to make inferences about morphological differences between different organisms. However, the genetic mechanisms underlying these interactions are better suited for upper level biology students. 2. Scientific Teaching Themes: Explain how the Teaching Tools and Strategies relate to the Scientific Teaching Themes of: · Active Learning: How will students actively engage in learning the concepts? List and/or explain the active learning strategies that are used in the tool. For example, activities could include think-pairshare, clicker questions, group discussion, debate, etc. Include both in-class and out-of-class activities. The practical and visual implementation of key concepts serves as a pleasant method to help students grasp morphological differences in bacterial species of their choice. Integrating a qualitative visual category to the course will allow students to make assessments of phenotypes that were previously uncategorized. · Assessment: How will teachers measure learning? How will students self-evaluate their learning? List and/or explain the kinds of assessment tools used to measure how well students achieved the learning objectives. For example, assessments might be clicker questions, forced choice questions, exams, posters, etc. This laboratory technique is a good way to assess practical reasoning and hypothesis construction in biology students. Lab reports of their observations would be assessed through their construction of logical interpretations of results as well as their capacity to grow plates that were relatively contaminant free. The replication of these results would be an important factor to assess within their given framework of funds. · Inclusive Teaching: How is the Teaching Tool and Strategy designed to include all participants and acknowledge the value of diversity in science? List and/or explain how the Teaching Tool and Strategy is inclusive and how it leverages diversity in the classroom and beyond. For example, the Teaching Tool and Strategy may use multiple senses and provide examples of scientists from different backgrounds. Visual strategies enhance discussion within the classroom and between peers outside of regular class hours. Due to the simplicity of the skills required to set up a swarming plate, it can be incorporated into classes with varying degrees of complexity. The total time involved in setting up these plates is also minimal, with only short bursts of time allocated to image the changes in growth. The visual appeal of the patterns that emerge from swarming draws students who may not normally be as inclined toward laboratory work. This would encourage sharing of results and peer reviews of observations. v Subheadings: can be included within the sections above to increase readability and clarity. Acknowledgments Begin the Acknowledgements on a new page. The acknowledgements can be multiple paragraphs. Dr. Brooke Jude References Begin the References on a new page. * Cite references in the text using superscript Arabic numbers. Use commas to separate multiple citation numbers. Superscript numbers are placed outside periods and commas and inside colons and semicolons. 1. Begin the reference list on a new page. The reference list is comprehensive and spans the text, figure captions and materials. 2. Number references in the order in which they appear in the text. Follow ASM style and abbreviate names of journals according to the journals list in NCBI. List all authors and/or editors up to 6; if more than 6, list the first 3 followed by “et al.” Note: Journal references should include the issue number in parentheses after the volume number. Examples of reference style: 1. Knight JK, Wood WB. 2005. Teaching more by lecturing less. Cell Biol Educ. 4(4):298-310. 2. Samford University. How to get the most out of studying: A video series. www.samford.edu/how-to-study/. Accessed August 20, 2013. 3. Handelsman J, Miller S, Pfund C. 2006. Scientific Teaching. New York, NY:W.H. Freeman. 3. Please add notes to the end of the reference list; do not mix in references with explanatory notes. Figure and Table Legends Begin legends on a new page. * The actual figures, tables, and supplemental materials are uploaded as separate documents and should not be included in this text file. Figures: Figure. A sample model of techniques used during for the Swarming Motility Assay and the same plates with iodabacter sp. growth after ~72 hours and after 10 days of growth at room temperature. Supplementary Materials: Swarming Agar composition: R2A Swarming media: The R2A swarming media utilizes 0.5% agar (5g/L), Proteose peptone (0.5g/L) , Casamino acids (0.5g/L), Yeast extract (0.5g/L), Dextrose (0.5g/L), Soluble starch (0.5g/L), Dipotassium phosphate (0.3g/L), Magnesium sulfate 7H2O (0.05g/L), Sodium pyruvate (0.3g/L) at a final pH of 7 ± 0.2 at 25 °C LB swarming media: The LB swarming media consists of 0.5% agar (5g/L), Tryptone (10g/L), NaCl (5g/L), and yeast extract (5g/L) 1% Tryptone swarming media: The tryptone swarming media consists of 0.5% agar (5g/L) and tryptone (10g/L)