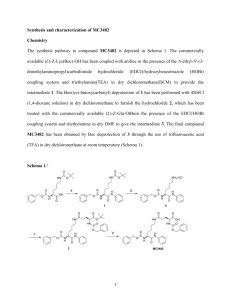
Abstract The direct and catalytic photodegradations of two toxic
advertisement

Abstract The direct and catalytic photodegradations of two toxic pesticides (Chlorpyrifose and Thiamethoxam) were investigated under the artificial (UV and Visible) source of light and solar radiation (Sun light). The direct photodegradation was tested and appeared not to be an effective method for mineralizing these two pesticides. The catalytic photodegradation processes were studied in aqueous suspensions of different semiconductors (TiO2, ZnO and TiO2 +ZnO). It was found that an increase in the concentration of catalysts resulted in enhanced activity. The efficiencies of catalytic photodegradation in all systems were compared and the results indicate that the catalytic activity of TiO2 for Chlorpyrifose and Thiamethoxam are the most effective photocatalysts. UV-Visible spectroscopy was used to monitor changes in pesticides concentration. The data obtained by UV-Visible spectroscopy was used to determine the kinetic parameters of the degradation of two pesticides. It was found that the degradation of Chlorpyrifose and Thiamethoxam in all experiments , followed an pseudo-first order reaction. The influences of various Factors on the catalytic photodegradation were examined. A series of experiments were carried out at different temperatures ranging from 20ºC to 50ºC. The activation energies obtained in these systems are: 18.451 to 27.75 kJ/mole. The another effect on catalytic degradation of the pesticides was performed, using three kinds of UV-light lamps: the first was emitting the UV-light without a wavelength selector and the others were lamps emitting the selected UV-light in the wavelength of 254 and 365 nm. The catalytic photodegradation of two pesticides was studied with the most efficient system under UV-radiation with TiO2 as catalyst in different natural waters (lake, river, ground and drinking waters). The effects of these types of waters on the kinetic parameters have been investigated.