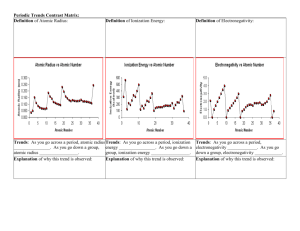
Periodic Trends
advertisement
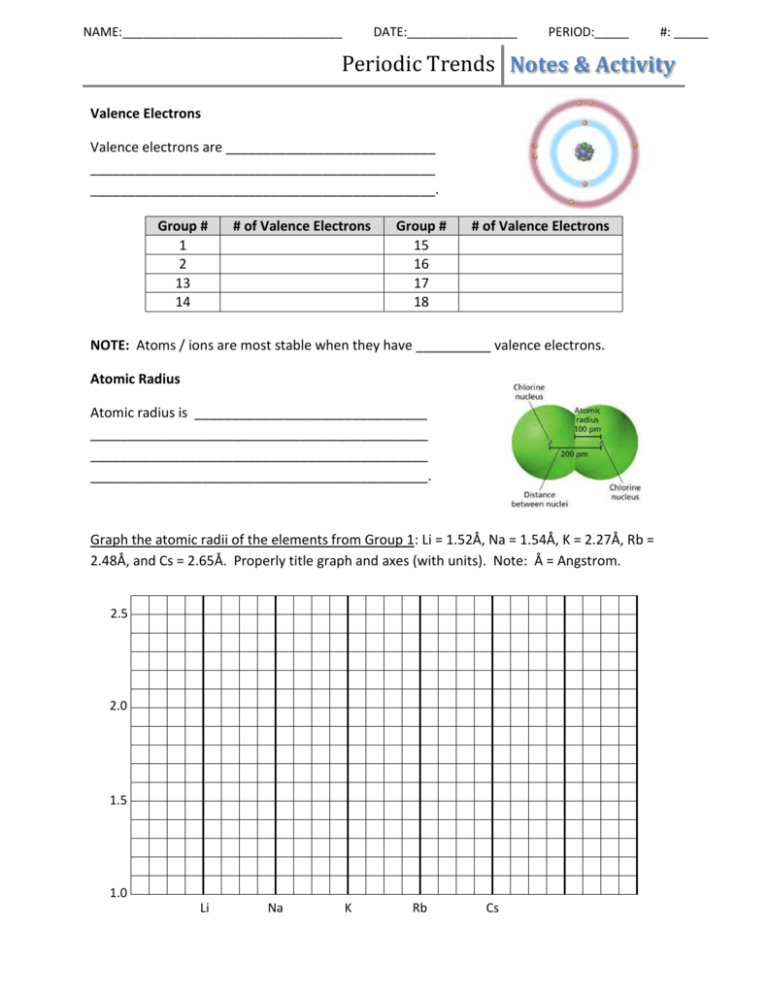
NAME:________________________________ DATE:________________ PERIOD:_____ #: _____ Periodic Trends Notes & Activity Valence Electrons Valence electrons are ____________________________ ______________________________________________ ______________________________________________. Group # 1 2 13 14 # of Valence Electrons Group # 15 16 17 18 # of Valence Electrons NOTE: Atoms / ions are most stable when they have __________ valence electrons. Atomic Radius Atomic radius is _______________________________ _____________________________________________ _____________________________________________ _____________________________________________. Graph the atomic radii of the elements from Group 1: Li = 1.52Å, Na = 1.54Å, K = 2.27Å, Rb = 2.48Å, and Cs = 2.65Å. Properly title graph and axes (with units). Note: Å = Angstrom. 2.5 2.0 1.5 1.0 Li Na K Rb Cs Periodic Trends Notes & Activity Q: What is the trend in atomic radius as you move down a group? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Actual Reason Graph the atomic radii of the elements from Period 3: Na = 1.54Å, Mg = 1.36Å, Al = 1.25Å, Si = 1.17Å, P = 1.10Å, S = 1.04Å, and Cl = 0.99Å. Properly title graph and axes (with units). 2.0 1.5 1.0 0.5 Na Mg Al Si P S Cl Q: What is the trend in atomic radius as you move right across a period? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Actual Reason Periodic Trends Notes & Activity Ionization Energy Ions are atoms that have a positive or negative charge due to loss or gain of electrons. Ionization energy is ______________________________________________________________ _____________________________________________________________________________. A + Energy → _________________ Graph the ionization energies of the elements from Group 2: Be = 215, Mg = 176, Ca = 141, Sr = 131, Ba = 120, and Ra = 122. Properly title graph and axes (with units). Notes: units are kcal/mol. 250 200 150 100 Be Mg Ca Sr Ba Ra Q: What is the trend in ionization energy as you move down a group? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Actual Reason Periodic Trends Notes & Activity Graph the ionization energies of the elements from Period 2: Li = 125, Be = 215, B = 191, C= 260, N= 336, O= 314, and F= 402. Properly title graph and axes (with units). 400 300 200 100 Li Be B C N O F Q: What is the general trend in ionization energy as you move right across a period? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Actual Reason Periodic Trends Notes & Activity Electronegativity Electronegativity is ______________________________________________________________ ______________________________________________________________________________ Graph the electronegativities of the elements from Group 7: F = 4.0, Cl = 3.2, Br = 2.9, and At = 2.2. Properly title graph and axes. Note: electronegativity has no units. I = 2.7, 4 3 2 1 F Cl Br I At Q: What is the trend in electronegativity as you move down a group? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Actual Reason Periodic Trends Notes & Activity Graph the electronegativities of the elements from Period 2: Li = 1.0, Be = 1.5, B = 2.0, 2.6, N = 3.1, O = 3.5 and F = 4.0. Properly title graph and axes. C= 4 3 2 1 Li Be B C N O F Q: What is the trend in electronegativity as you move right across a period? A: ____________________________________________________________________________ Q: Why does this trend occur? Prediction Trends Summary Actual Reason Periodic Trends Notes & Activity Application Problems 1. What would have a greater atomic radius, phosphorus or bismuth? 2. What would have a smaller atomic radius, silver or strontium? 3. What would have a greater atomic radius, tin or chlorine? 4. Arrange the following elements from largest to smallest atomic radii: Li, O, C, K, Cs and F. 5. What would have a smaller ionization energy, titanium or zinc? 6. What would have a greater ionization energy, magnesium or barium? 7. What would have a greater ionization energy, sodium or rubidium? 8. Arrange the following elements from smallest to largest ionization energy: Li, O, C, K, Cs and F. 9. What would have a smaller electronegativity, carbon or lead? 10. What would have a greater electronegativity, tellurium or palladium? 11. What would have a smaller electronegativity, chlorine or mercury? 12. Arrange the following elements from smallest to largest electronegativity: Li, O, C, K, Cs and F.