DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS
advertisement
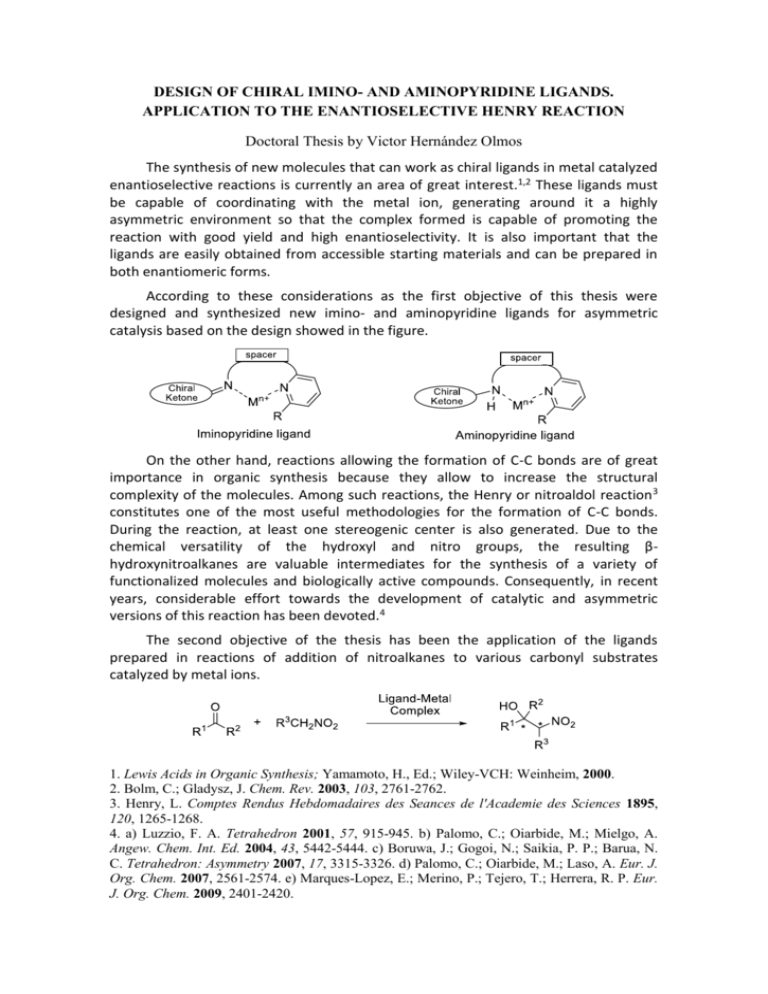
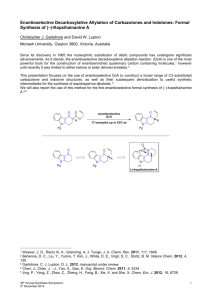
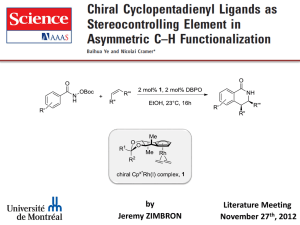
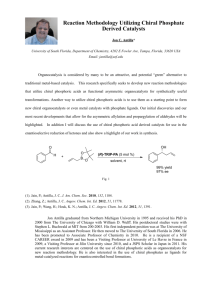
DESIGN OF CHIRAL IMINO- AND AMINOPYRIDINE LIGANDS. APPLICATION TO THE ENANTIOSELECTIVE HENRY REACTION Doctoral Thesis by Victor Hernández Olmos The synthesis of new molecules that can work as chiral ligands in metal catalyzed enantioselective reactions is currently an area of great interest.1,2 These ligands must be capable of coordinating with the metal ion, generating around it a highly asymmetric environment so that the complex formed is capable of promoting the reaction with good yield and high enantioselectivity. It is also important that the ligands are easily obtained from accessible starting materials and can be prepared in both enantiomeric forms. According to these considerations as the first objective of this thesis were designed and synthesized new imino- and aminopyridine ligands for asymmetric catalysis based on the design showed in the figure. On the other hand, reactions allowing the formation of C-C bonds are of great importance in organic synthesis because they allow to increase the structural complexity of the molecules. Among such reactions, the Henry or nitroaldol reaction 3 constitutes one of the most useful methodologies for the formation of C-C bonds. During the reaction, at least one stereogenic center is also generated. Due to the chemical versatility of the hydroxyl and nitro groups, the resulting βhydroxynitroalkanes are valuable intermediates for the synthesis of a variety of functionalized molecules and biologically active compounds. Consequently, in recent years, considerable effort towards the development of catalytic and asymmetric versions of this reaction has been devoted.4 The second objective of the thesis has been the application of the ligands prepared in reactions of addition of nitroalkanes to various carbonyl substrates catalyzed by metal ions. 1. Lewis Acids in Organic Synthesis; Yamamoto, H., Ed.; Wiley-VCH: Weinheim, 2000. 2. Bolm, C.; Gladysz, J. Chem. Rev. 2003, 103, 2761-2762. 3. Henry, L. Comptes Rendus Hebdomadaires des Seances de l'Academie des Sciences 1895, 120, 1265-1268. 4. a) Luzzio, F. A. Tetrahedron 2001, 57, 915-945. b) Palomo, C.; Oiarbide, M.; Mielgo, A. Angew. Chem. Int. Ed. 2004, 43, 5442-5444. c) Boruwa, J.; Gogoi, N.; Saikia, P. P.; Barua, N. C. Tetrahedron: Asymmetry 2007, 17, 3315-3326. d) Palomo, C.; Oiarbide, M.; Laso, A. Eur. J. Org. Chem. 2007, 2561-2574. e) Marques-Lopez, E.; Merino, P.; Tejero, T.; Herrera, R. P. Eur. J. Org. Chem. 2009, 2401-2420.