Supplementary Material Head Movement Duration Healthy subjects
advertisement

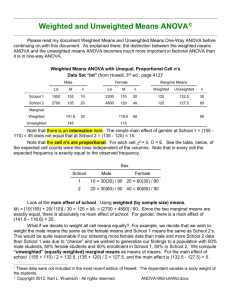
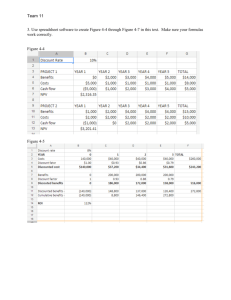
Supplementary Material Head Movement Duration Healthy subjects had near optimal values both in the unweighted (simulation: 450 ms (grey circle, Fig. 7A), experiment: 460.5±20.1 ms (blue circle), two-tailed t-test, P=0.615) and in the weighted condition (simulation: 570 ms (grey square), experiment: 586.4±22.4 ms (blue square), P=0.482). Vestibular-loss patients showed head movement durations of 548.4±106.4, 684.4±96.1 and 780.8±119.0 ms in unweighted, weighted early and late conditions, respectively. Cerebellar-ataxia patients showed head movement durations of 516.1±46.5, 561.8±55.8 and 640.2±51.2 ms in unweighted, weighted early and late conditions, respectively. rmANOVA revealed that durations were prolonged with weight in all groups (main effect of weight: F(1,21)=54.8, P<0.001, no interaction: F(2,21)=2.57, P=0.106). Over the course of the trials, only healthy subjects changed the head movement duration towards the new optimum (rmANOVA, interaction between within factor: early or late trials; between factor: group, F(2,21)=3.51, P=0.048). Peak Head Velocity Healthy subjects had near optimal values for peak head velocity both in the unweighted (simulation: 208.0°/s (Fig. 7B, grey circle), experiment: 201.6±14.0°/s (blue circle); P=0.661) and in the weighted condition (simulation: 144°/s (grey square), experiment: 134.0±10.4°/s (blue square); P=0.378). Vestibular-loss patients showed peak head velocity of 168.9±31.2, 97.6±15.5 and 91.6±8.6°/s in unweighted, weighted early and late conditions, respectively. Cerebellar-ataxia patients showed peak head velocity of 166.2±7.1, 102.3±13.1 and 117.8±11.2°/s. A rmANOVA (within factor: weight; between factor: group) revealed a main effect of weight F(1,21)=52.0, P<0.001 and no interaction (F(2,21)=0.90, P=0.421). The difference between the observed peak head velocity and the optimal ones were not significantly different in the unweighted and weighted 1 conditions. Over the course of the trials only cerebellar-ataxia patients (each of the nine) changed the head movement duration towards the new optimum (rmANOVA, interaction between within factor: early or late trials; between factor: group, F(2,21)=3.57, P=0.046). Head Contribution Healthy subjects had near optimal values for head contributions both in the unweighted (simulation: 29° (Fig. 8B, light grey circle), experiment: 27.7±1.5° (blue circle); P=0.412) and in the weighted condition (simulation: 25° (light grey square), experiment: 22.0±2.2° (blue square); P=0.148). Vestibular-loss patients showed head contributions of 22.2±4.5, 14.1±4.1 and 12.3±3.1° in the unweighted, and weighted early and late conditions, respectively. Simulations for additional 5° undershoot showed that head oscillations should be reduced to 24° and 18° for unweighted and weighted conditions, respectively (dark grey circle and square). This reflects the behaviour of cerebellar-ataxia patients. Their head contributions were 21.4±1.8, 11.3±1.4 and 14.6±1.1° in the unweighted, and weighted early and late conditions, respectively. A rmANOVA (within factor: weight; between factor: group) revealed main effects of weight (F(1,21)=54.8, P<0.001) and group (F(2,21)=49.3, P<0.001, post-hoc Scheffé, P(H,CA)=0.046) on head contributions without any interaction (F(2,21)=1.17, P=0.33). The difference between the observed head contribution and the optimal values was significantly lower in healthy subjects than in vestibular-loss patients in the weighted condition (P=0.048). None of the subjects changed the head contribution over the course of the trials (rmANOVA, within factor: early or late trials, F(1,21)=1.59, P=0.221; no interaction with group factor, F(2,21)=1.78, P=0.193). Eye Movement Duration The simulation results indicated that eye movement duration has to be updated when the head moment of inertia is increased (simulations, from unweighted: 200 ms to weighted: 2 260 ms). Healthy subjects had eye movement durations of 217.8±7.1 ms in the unweighted condition (the difference between simulations and experiment was 17.8±7.1 ms, P=0.033). Increasing the head moment of inertia initially prolonged eye movement duration (weighted early trials: 266.9±9.9 ms, P<0.001). There was no change over the course of the trials (weighted late trials: 265.2±10.8 ms, P=0.692) because the values were already close to simulations (P=0.502). Vestibular-loss patients showed eye movement durations of 236.1±21.4, 307.1±35.9 and 299.1±39.4 ms in the unweighted, and weighted early and late conditions, respectively. Cerebellar-ataxia patients displayed eye movement durations of 214.1±13.7, 233.2±17.2 and, 255.6±28.2 ms. A rmANOVA (within factor: weight; between factor: group) revealed a main effect of weight (F(1,21)=31.3, P<0.001) and no interaction (F(2,21)=0.57, P=0.631). The difference between the observed eye movement duration and the optimal values were larger in vestibular-loss patients than in the other groups in both the unweighted (P=0.050) and the weighted condition (P=0.015). There was no change in the eye movement duration over the course of the trials (rmANOVA, within factor: early or late trials F(1,21)=0.21, P=0.653; no interaction F(2,21)=1.01, P=0.382). In healthy subjects and cerebellar-ataxia patients, this can be attributed to the fact that initial values were already close to optimal. Effect of Age in Cerebellar Ataxia Patients Groups were age-matched. To make sure that the non-significant higher age of cerebellar ataxia patients did not affect the change of parameters throughout the trials, we grouped the cerebellar-ataxia patients into young (n=5, age<60) and old (n=4, age>60) groups. There was no main effect of age on head oscillation ratio, gaze fluctuations, eye movement duration, head movement duration, head contribution, and peak head velocity (all F(1,7)<2.3, all P>0.17). The grouping factor age did not interact with the within factor: early or late trials for any of the measures (all F(1,7)<3.76, all P>0.09). 3 Plant Dynamics Eye and head plant dynamics were modelled by third order differential equations. The control signal ue drives the eye plant that is governed by the following differential equation: xe + t 1t 2 + t 1t e + t 2t e t +t +t 1 1 xe + 1 2 e xe + xe = ue t 1t 2t e t 1t 2t e t 1t 2t e te (1) where xe , xe , xe , xe are eye position, velocity, acceleration and jerk, respectively. The plant parameters t 1, t 2 , t e were chosen as 224, 13 and 10 ms (Harris and Wolpert, 1998; Robinson et al., 1986; Tanaka et al., 2006; van Beers, 2008). The control signal uh drives the head plant that is governed by the following differential equation: xh + ( b K b K 1 + )xh + ( + )xh + x= u th I I thI thI thI h 1 (2) where xh , xh , xh , xh are head position, velocity, acceleration and jerk, respectively. The plant parameters related to viscosity and stiffness terms were b = 0.3 N m s/rad, K = 2.077 N m/rad, respectively (Peng et al., 1996; Tangorra et al., 2003). The additional time constant h 100 ms was used to account for low-pass dynamics of the muscle activations (Peng et al., 1996, Sağlam et al., 2011). The head moment of inertia was 0.0148 kg m2 for the unweighted (Peng et al., 1996) and 0.0488 kg m2 for the weighted condition (Lehnen et al., 2009a). The plant parameters for both eye and head were the same as in our previous study (Sağlam et al., 2011). Optimal Control Model Optimal control signals ue and uh were selected based on the criterion that the sum of the consequences of signal-dependent noise (SDN) and constant noise (CN) are minimized. The eye movements during the gaze shift were separated into two phases: i) From the 4 onset of the movement until the eye reaches maximum eccentricity (eye movement duration) and ii) from the maximum eccentricity until the head stops. Therefore, the total cost function includes two SDN ( J SDN ,e1 , J SDN ,e2 ) and two CN ( J CN ,e1 , J CN ,e2 ) terms for the eye and one SDN ( J SDN ,h ) and CN ( J CN ,h ) term for the head: JTotal = J SDN ,e1 + J SDN ,e2 + J SDN ,h + J CN ,e1 + J CN ,e2 + J CN ,h (3) SDN-related cost terms for the head movement and for the two phases of the eye movement were weighted by h , e1 and e 2 as in Sağlam et al. (2011). Similarly, CNrelated cost terms were weighted by b h , b e1 and b e2 . The full optimal control model contains 3 free parameters (e1, e2, and h penalizing CN) that determine the compromise between SDN- and CN-related costs. The parameters were chosen to fit the observed kinematic parameters in the unweighted condition. All fixed parameters were unchanged to the previous model (Sağlam et al., 2011) except for the parameter h that penalized SDN-related head movement cost, which was increased 1.75-fold to better match the data. The model parameters were selected as a e1 = a e2 = 1 , a h = 1.75 , be1 = 2.95 , be2 = 0.45 , b h = 0.72 by fitting the observed kinematic parameters in the unweighted condition. To compute the cost (positional variance) of a movement with a given kinematic parameter set (e.g., specific eye and head durations and contributions), we simulated a movement with those kinematic parameters. Eye and head control signals ue and uh , which determined the whole extent of the movements for those kinematic parameters, were computed according to the Pontryagin's Minimum Principle as previously done (van Beers, 2008; Sağlam et al., 2011). This enabled making a search in the kinematic parameter space. By finding the optimal kinematic parameters, we found the optimal eye and head movements for a gaze shift that minimized the cost function (Eq.3). 5 References Robinson DA, Gordon JL, Gordon SE. A model of the smooth pursuit eye movement system. Biol Cybern 1986; 55: 43-57. Tanaka H, Krakauer JW, Qian N. An optimization principle for determining movement duration. J. Neurophysiol. 2006; 95: 3875-3886. Tangorra JL, Jones LA, Hunter IW. Dynamics of the human head-neck system in the horizontal plane: joint properties with respect to a static torque. Ann Biomed Eng 2003; 31: 606-20. 6