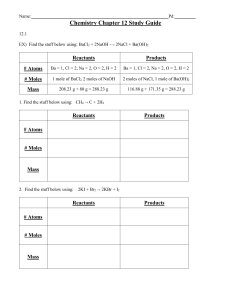
Name Date Class Worksheet #3 Chapter 12 – Stoichiometry Limiting
advertisement

Name ___________________________________ Date ______________________ Class ______________________ Worksheet #3 Chapter 12 – Stoichiometry Limiting Reactants When chemicals are mixed together to undergo a reaction, they are often mixed in stoichiometric quantities, that is, in exactly the correct amounts so that all of the reactants “run out” and are used up at the same time. A good analogy is baking chocolate chip cookies. Imagine you want to make as many cookies as you can. You have lots of flour, eggs, sugar, and vanilla, but you only have 1 package of chocolate chips. Since the recipe for a dozen cookies calls for 1 package of chocolate chips, you can only make four dozen cookies. In this case, you had an excess of all the other ingredients and what determined the amount of cookies (product) you could make was the amount of chocolate chips. In this example the chocolate chips were the limiting reactant. Now, let’s look at a chemical example. In the following reaction of making ammonia, two moles of ammonia would be produced by reacting one mole of nitrogen and three moles of hydrogen. N2 (g) + 3H2 (g) → 2NH3 (g) 1 mole 3 moles 2 moles Now consider an example where the reactants are not in the correct stoichiometric quantities: N2 (g) + 3H2 (g) → 2NH3 (g) 2 moles 3 moles ? moles Since you now have twice as much nitrogen, you will use all of the hydrogen and some nitrogen will be left over (1 mole) so hydrogen limits the amount of product produced. We will call hydrogen the limiting reactant (reagent) because the reaction will stop when you run out of hydrogen. Consider another example where the reactants are not in the correct stiochiometric quantities: N2 (g) + 3H2 (g) → 2NH3 (g) 1 mole 4 moles ? moles Since you have more hydrogen than is needed, you will use up all of the nitrogen and some of the hydrogen will be left over (1 mole) so nitrogen limits the amount of product produced and we call it the limiting reactant. The reaction will stop when you run out of nitrogen. How do you know that a problem is a limiting reactant problem and how do you solve it once you have identified it? The answer to the first question is you know when you are given a limiting reactant problem when you are given a stoichiometry problem and the masses of both reactants are given. In order to solve the problem you must follow the following steps. 1. Determine the knowns (what you know/information given) and unknown (what you want to know) and record them above the equation. 2. Using “what you know” (known masses) determine TWO possible masses produced. Using the process learned in the last two worksheets. 3. Compare the two masses and see what reactant is limiting. The limiting reactant runs out first, SO it limits how much product is produced, therefore the smaller amount of product is produced from the limiting reactant. Example: The reaction between solid white phosphorus and oxygen produces tetraphosphorus decoxide. Determine the mass in grams of P4O10 formed if 25.0 grams of phosphorus (P4) and 50.0 grams of oxygen are combined. Balanced Equation: P4 + 5O2 → P4O10 Solve the following problems showing all work for full credit. 1. The reaction between solid sodium and iron (III) oxide is one in a series of reactions that inflate an automotive air bag. The two products are sodium oxide and iron. If 100.0 grams of sodium reacts with 100.0 grams of Fe2O3, determine the mass of solid iron produced. (6Na + Fe2O3 → 3Na2O + 2Fe) 2. Photosynthesis reactions in green plants use carbon dioxide and water to produce glucose (C6H12O6) and oxygen. Calculate the mass of glucose produced if 88.0 grams of carbon dioxide and 64.0 grams of water are available to a plant growing in sunlight. (6CO2 + 6O2 → C6H12O6 + 6O2) 3. Disulfur dichloride is used to vulcanize rubber, a process invented by Charles Goodyear. The process makes rubber harder, stronger, and less likely to become soft when hot or brittle when cold. In the production of disulfur dichloride, molten sulfur reacts with chlorine gas to the following equation: S8 + 4Cl2 → 4S2Cl2. If 200.0 grams of sulfur reacts with 100.0 grams of chlorine, what mass of disulfur dichloride is produced? 4. Iron is obtained commercially in a blast furnace by the reaction of hematite (Fe2O3) with carbon monoxide. The two products are iron and carbon dioxide. How many grams of iron are produced if 75.0 grams of hematite react with 40.0 grams of carbon monoxide? (Fe2O3 + 3CO → 2Fe + 3CO2) 5. Hydrogen gas can be produced by the reduction of magnesium metal with hydrochloric acid. Mg + 2HCl → MgCl2 + H2. Identify the limiting reactant when 6.0 grams HCl reacts with 5.0 grams Mg.