lab 02 - observing polar bonds
advertisement

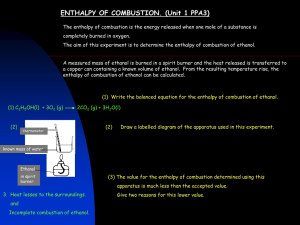
Name _________________________ Date _______ Period ___ Group ___ Lab Activity: Observing Polar Bonds Safety: This lab utilized ethanol which is flammable and boiling water. Secure loose clothing and take appropriate precautions. Background: Surface tension in a liquid varies with the degree of polarization in the molecules. Water is significantly more polar than ethanol. Purpose: To observe properties of polar molecules. Materials: Petri dish full of water Petri dish full of ethanol Paper clip Disposable pipet Capillary tube Penny Procedure: 1. Try to float a penny on water 2. Try to float a penny on ethanol 3. Using a plastic pipet, observe how many drops of water you can place on penny before the water pours over the sides. Repeat with old and new pennies. 4. Using a plastic pipet, observe how many drops of ethanol you can place on penny before the ethanol pours over the sides. Repeat with old and new pennies. 5. Observe the capillary action of cold water. 6. Observe the capillary action of hot water. 7. Observe the capillary action of ethanol Analysis: Explain why the observed differences occurred between water and ethanol Name _________________________ Date _______ Period ___ Group ___ Lab Activity: Observing Polar Bonds Safety: This lab utilized ethanol which is flammable and boiling water. Secure loose clothing and take appropriate precautions. Background: Surface tension in a liquid varies with the degree of polarization in the molecules. Water is significantly more polar than ethanol. Purpose: To observe properties of polar molecules. Materials: Petri dish full of water Petri dish full of ethanol Paper clip Disposable pipet Capillary tube Penny Procedure: 1. Try to float a penny on water 2. Try to float a penny on ethanol 3. Using a plastic pipet, observe how many drops of water you can place on penny before the water pours over the sides. Repeat with old and new pennies. 4. Using a plastic pipet, observe how many drops of ethanol you can place on penny before the ethanol pours over the sides. Repeat with old and new pennies. 5. Observe the capillary action of cold water. 6. Observe the capillary action of hot water. 7. Observe the capillary action of ethanol Analysis: Explain why the observed differences occurred between water and ethanol