Special Review Checklist for Non-Sponsored Projects 6 Sept 2012
advertisement
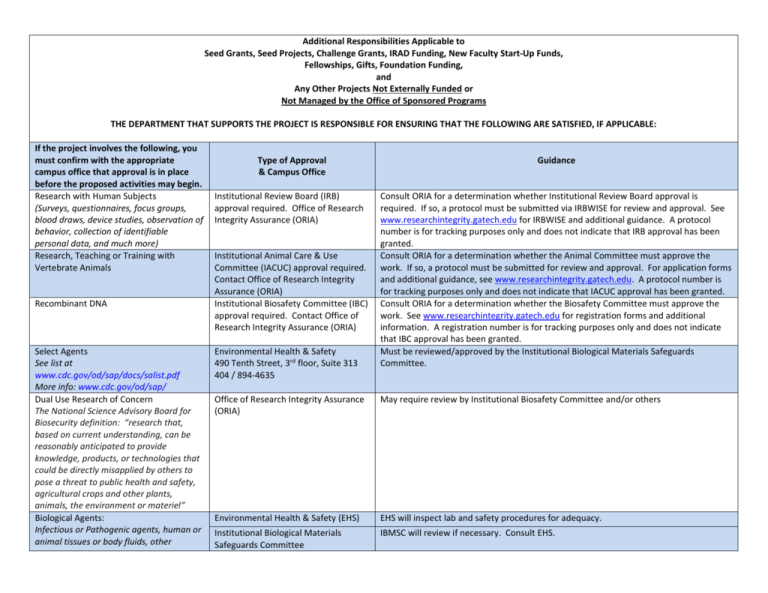
Additional Responsibilities Applicable to Seed Grants, Seed Projects, Challenge Grants, IRAD Funding, New Faculty Start-Up Funds, Fellowships, Gifts, Foundation Funding, and Any Other Projects Not Externally Funded or Not Managed by the Office of Sponsored Programs THE DEPARTMENT THAT SUPPORTS THE PROJECT IS RESPONSIBLE FOR ENSURING THAT THE FOLLOWING ARE SATISFIED, IF APPLICABLE: If the project involves the following, you must confirm with the appropriate campus office that approval is in place before the proposed activities may begin. Research with Human Subjects (Surveys, questionnaires, focus groups, blood draws, device studies, observation of behavior, collection of identifiable personal data, and much more) Research, Teaching or Training with Vertebrate Animals Recombinant DNA Select Agents See list at www.cdc.gov/od/sap/docs/salist.pdf More info: www.cdc.gov/od/sap/ Dual Use Research of Concern The National Science Advisory Board for Biosecurity definition: “research that, based on current understanding, can be reasonably anticipated to provide knowledge, products, or technologies that could be directly misapplied by others to pose a threat to public health and safety, agricultural crops and other plants, animals, the environment or materiel” Biological Agents: Infectious or Pathogenic agents, human or animal tissues or body fluids, other Type of Approval & Campus Office Institutional Review Board (IRB) approval required. Office of Research Integrity Assurance (ORIA) Institutional Animal Care & Use Committee (IACUC) approval required. Contact Office of Research Integrity Assurance (ORIA) Institutional Biosafety Committee (IBC) approval required. Contact Office of Research Integrity Assurance (ORIA) Environmental Health & Safety 490 Tenth Street, 3rd floor, Suite 313 404 / 894-4635 Guidance Consult ORIA for a determination whether Institutional Review Board approval is required. If so, a protocol must be submitted via IRBWISE for review and approval. See www.researchintegrity.gatech.edu for IRBWISE and additional guidance. A protocol number is for tracking purposes only and does not indicate that IRB approval has been granted. Consult ORIA for a determination whether the Animal Committee must approve the work. If so, a protocol must be submitted for review and approval. For application forms and additional guidance, see www.researchintegrity.gatech.edu. A protocol number is for tracking purposes only and does not indicate that IACUC approval has been granted. Consult ORIA for a determination whether the Biosafety Committee must approve the work. See www.researchintegrity.gatech.edu for registration forms and additional information. A registration number is for tracking purposes only and does not indicate that IBC approval has been granted. Must be reviewed/approved by the Institutional Biological Materials Safeguards Committee. Office of Research Integrity Assurance (ORIA) May require review by Institutional Biosafety Committee and/or others Environmental Health & Safety (EHS) EHS will inspect lab and safety procedures for adequacy. Institutional Biological Materials Safeguards Committee IBMSC will review if necessary. Consult EHS. biohazardous materials Office of Research Integrity Assurance Physical Agents Includes chemicals, sharps, thermal agents, lasers, radiation Environmental Health & Safety Materials Being Transferred Between Ga Tech and Another Party Industry Collaboration, International Contracts, and Innovation Commercialization (IC3) Export of Any Information, Technology, or Materials to Another Country Involves a foreign sponsor or collaborator; or will be performed in whole or in part outside of the us; involves travel outside of US; publication restriction; or foreign nationals not allowed to participate Activity involves the use of specific results or intellectual property from previous research. Sharing of Proprietary Information Office of Research Integrity Assurance Export review required. Office of Legal Affairs or Office of Research Integrity Assurance Export review required. Industry Collaboration, International Contracts, and Innovation Commercialization (IC3) Office of Legal Affairs Consult IC3 prior to disclosing information. Conflict of Interest Office of Conflict of Interest Management Consult the previously listed office(s) for guidance. Researcher has joint GT-Emory appointment and will conduct research activities at Emory, not at GT. Researcher has GT appointment and will conduct research activities at another university, not at GT. Visiting scholar will conduct any activities at GT. September 6, 2012 Consult the previously listed office(s) for guidance. Office of Legal Affairs If human tissues/body fluids are indicated, consult Office of Research Integrity Assurance regarding IRB requirements. Chemicals, sharps, and thermal agents: Debbie Wolfe-Lopez, Chemical Safety Manager 404 / 385-2964 Lasers, Radiation: Nazia Zakir, Radiation Safety Officer 404 / 894-3605 ors@ors.gatech.edu May require a Materials Transfer Agreement (MTA), an agreement specifying that materials being made available by one party to another are for scientific work only and not for commercial use. No right of ownership or commercial use is transferred to the recipient of the materials. Cynthia Jackson 404 / 385-2723 May necessitate a Nondisclosure Agreement (NDA), which must be executed before information is exchanged. May necessitate a written, approved management plan Researcher must satisfy all applicable Ga Tech requirements mentioned herein. Deferral of IRB or IACUC approval to Emory may be appropriate. Consult ORIA. Researcher must satisfy all applicable Ga Tech requirements mentioned herein. Deferral of IRB or IACUC approval to the other entity may be appropriate, or that entity might defer to Georgia Tech. Consult ORIA. Visiting Scholar Agreement must be executed before any activities commence, and any all other applicable requirements must be satisfied.