Dual_Nature_of_Matter-Main
advertisement
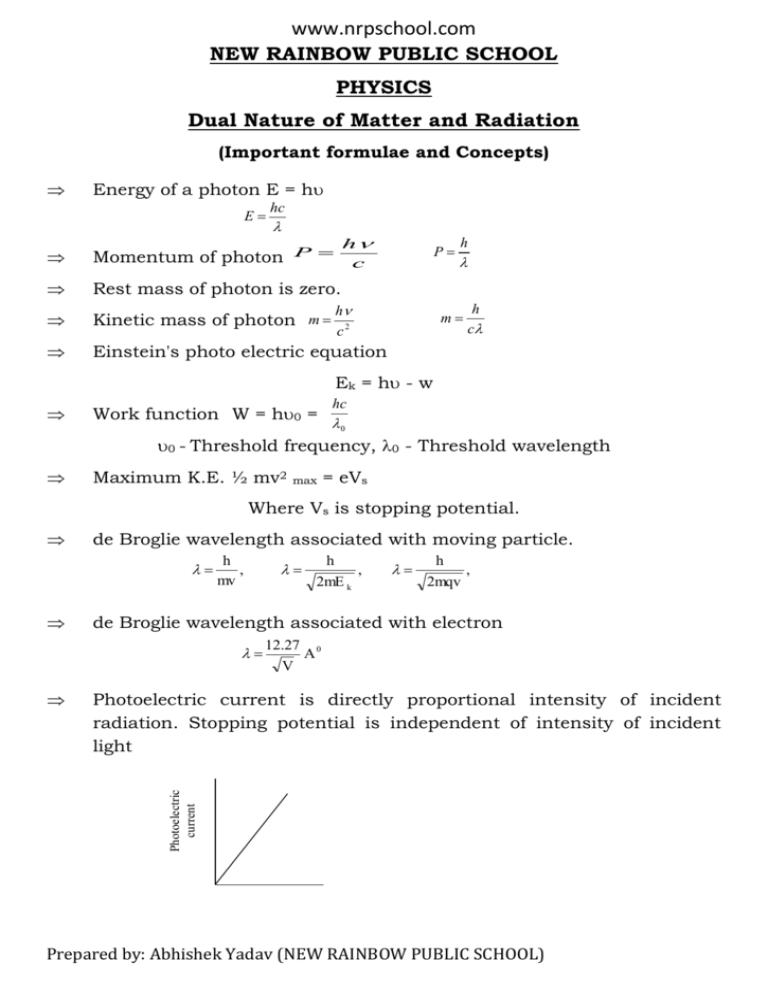
www.nrpschool.com NEW RAINBOW PUBLIC SCHOOL PHYSICS Dual Nature of Matter and Radiation (Important formulae and Concepts) Energy of a photon E = h E hc h c Momentum of photon P Rest mass of photon is zero. Kinetic mass of photon m Einstein's photo electric equation P h c2 h m h c Ek = h - w Work function W = h0 = hc 0 0 - Threshold frequency, 0 - Threshold wavelength Maximum K.E. ½ mv2 max = eVs Where Vs is stopping potential. de Broglie wavelength associated with moving particle. h , mv h 2mE k , h 2mqv , de Broglie wavelength associated with electron 12.27 V A0 Photoelectric current is directly proportional intensity of incident radiation. Stopping potential is independent of intensity of incident light Photoelectric current Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com Photoelectric current Intensity of incident light 3I 2I I 2I Potential difference (V) Stopping potential is directly proportional to frequency of incident radiation. Maximum kinetic energy of photoelectrons is directly proportional to frequency of incident radiation as well stopping potential Intensity of light never affects stopping potential as well as maximum kinetic energy of photoelectrons. When frequency and intensity of incident light are kept field and photo metal is changed. We observe that stopping potential (Vs) versus frequency () graphs are parallel straight lines, cutting frequency axis at different points. Metal 1 Metal 2 Vs 0 01 02 Frequency Work function is defined as the minimum, energy required to free an electron from its metallic bonding is called work function. It is denoted by W. W = h0 Threshold frequency: The minimum frequency of incident light which is just capable of ejecting electrons from a metal is called the threshold frequency. It is denoted by 0 Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com Stopping potential:- The minimum retarding potential applied to anode of photoelectric tube. Which is just capable of stopping photoelectric current is called the stopping potential. It is denoted by V0 (or Vs) Einstein's photoelectric equation. Ek = h - W Or ½ m2 max = h - ho Vs - Stopping potential Or eV0 = h - h0 Ek - max. Kinetic energy of electron Vs Other symbols have their. h h 0 e e Usual meaning. The slope of Ek Versus graph is h. The slope of Vs Versus graphs ( h/e) is Where symbol have their usual meaning. QUESTIONS FOR SUPPORTIVE LEARNERS 1. The wave length of electro magnetic radiation is doubled; How will the energy of a photon change ? Ans. E hc 1 Clearly when wave length is doubled, the energy of photon is hauled. 2. What is the stopping potential applied to a photo cell is the maximum. Kinetic energy of photo electron to 5 eV? Ans. Ek = eVo 5ev = eVo Or Vo = 5 v The stopping potential V0 = 5 volt (negative) 3. Two metal A and B have work function 2 eV and 4 eV respectively which metal has a lower threshold wavelength for photoelectric effect? Ans. Work function W hc hc 1 0 0 w w Clearly metal B has lower threshold wavelength. Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 4. Work function of sodium is 2.3 eV. Does sodium show photo electric emission for light of wavelength 6800A0 Ans. 0 hc 6.6 10 -34 3 10 8 5380 A 0 19 w 2.3 1.6 10 Thus 0 < , No photo electric emission will take place. 5. Two beams one of red light and other of blue light of the same intensity are incident on a metallic surface to emit photoelectric which one of the two beams emits electrons of greater kinetic energy? Ans W 0 hc 0 hc 1 w w The photon of blue light emits electrons of greater kinetic energy than that of red light, becomes larger wavelength of red that blue. 6. Ultraviolet light is incident on two photosensitive materials having work functions W1 and W2 (W1 > W2). In which case will the kinetic energy of the emitted electron greater? Why? Ans. K.E. for metal of Work function W2 Will be greater. As Ek = h - W. small as work function greater K.E. 7. Ultraviolet radiations of deferent frequencies 1 and 2 are incident on two photo sensitive, materials having work functions W1 and W2 (W1 > W2) respectively. The kinetic energy of the emitted photo electrons is same in both the cases. Which one of the two relations will be of the higher frequency? Ans. According to Einstein's photoelectric equation, Ek = h - w As Ek is same, h1 - w1 = h1 - w2 h1 - w1 = h1 - w2 w1 w2 h Or 1-2 = , As w1 > w2, 1 > 2 So frequency of radiation 1 is higher. 8. With what purpose famous Davison Germer experiment with electrons performed? Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com Ans. Davison- Germer experiment was performed to verify wave native of electrons. 9. Name the experiment which establishes the wave native of particle. Ans. Davison -Germer experiment. 10. If the potential difference used to accelerate electron is tripled, by what factor does de Broglie wavelength of electrons beam change. Ans. De Broglie wavelength becomes 1 3 time. h 2mev 11. An electron, an alpha particle and a proton have the same kinetic energy, which one of these particles has (i) the shortest and (ii) the Ans. largest, de, Broglie wavelength? h 1 2mEk m For same kinetic energy. (i) Out of given particle, the mass of alpha particle is maximum so de Broglie wavelength associated with alpha particle is shortest. (ii) As man of electron is least, so electron has largest de Broglie. wavelength. 12. An electron and a proton have the same kinetic energy, which one of the two has the larger wavelength and why? Ans: An electron has larger wave length as its mass is small. 1 m 13. An electron and a proton have the same de Broglie wave length associated with them. How are their kinetic energies related to each other? Ans. Given e = p h 2m e E e h 2m p Fp h 2mE k Ee mp E p me 1840 i.e. K.e. of electron = 1840 K.e. of proton. 14. A proton and a deuteron have the same velocity, what is the ratio of their de Broglie wavelengths? 1 m for the same velocity Ans. As p m d 2m p d m p mp Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 15. An electron and alpha particle have the same kinetic energy. How are the de Broglie. wavelength associated with their related? 16. How will the photoelectric cement change on decreasing the wavelength of incident radiation for a given photo sensitive material? Ans. It is independent of the frequency of incident light or wavelength of incident light? e m me e 86.5 4m p me e 1872 4 17. What is the de Broglie wavelength (in A0) associated with an electron accelerated through potential difference of 100 volt. Ans. 18. 12.27 0 A v 12.27 0 A 1.22 A 0 100 de-Broglie wavelength associated with an electron accelerated through a potential difference V is . What will be the de-Broglie wavelength when the accelerating p.d. is increased to 4V? 1 1 V 4 , 2 2 V1 2 1 2 v 2 Short Answer Questions19. For a photosensitive surface, threshold wavelength is 0. Does photoemission if the wavelength radiation is (i) more than 0 and (ii) less than 0? Justify your answer. Ans. Energy of a photon for emission of photoelectrons energy of photon >, work function or wavelength of incident photon should be less than, hc threshold wavelength 0 (i.e. < 0) E 20. Two metals and when illuminated with appropriate radiations emit photoelectrons. the work function of is higher than that . Which metal has higher valve of threshold frequency and why? Ans: Work function W = h0 W 0 So threshold frequency of 0 is higher than that of . Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 21. An particle and a proton are accelerated from state of rest through the same potential difference V. Find the ratio of de-Broglie wavelength associated with them? Ans: h 2mqv h , 2 4m p 2ev p h 2 4m p ev 1 : p 1 : 2 2 p 8 22. Two metals and have work function 2 ev and 4eV respectively which metal will emit electrons when irradiates with light of wavelength 400 nm and why? Ans: hc 6.6 10 -34 3 108 3.09 400 10 9 This is greater than work function of , but less than work function of so metal will emit electrons. 23. In a plot of photoelectric current versus anode potential, How does. (i) The saturation current vary with anode potential for incident radiations of different frequencies but same intensity. (ii) The stopping potential vary for incident radiations of different intensities but same frequency? (iii) Photoelectric current vary for different intensities bid same frequency of incident radiations? Justify your answer in each case. Ans. (i) In photoelectric affect the salvation current does not very with anode potential with incident radiation of different frequencies. The reason in that satiations current depends only on intensity of incident radiation because a single photon can eject a single electron, however large the frequency of radiation may be. (ii) obviously stopping potential is independent of intensity provided frequency remains unchanged, that is stopping potential does not vary with intensity of incident radiations. (iii) Photoelectric current increases with increase of intensity because increase in intensity of radiation means increase in umber of Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com incident photons As one photon ejects one electron, increase intensity cause increase in photoelectric current. 24. Show that Bohr's second postulate 'the electron revolves around the nucleus only in certain fixed orbit without radiating energy can be explained on the basis of do. Broglie hypothesis of wave nature of electron. Ans: The de Broglie wavelength Now for electron in orbit h mv 2r n (for nth oribit) nh 2r mv nh or mvr 2 This is Bohr's second postulate. As complete de-Broglie wavelength may be in certain fixed orbits. So non-radiating electron can be only in certain fixed orbit. 25. Red light however bright it is, can produce emission of electrons from a clean zinc surface, but even weak ultraviolet radiation can do so, why? Ans. The energy of photon of U.V. light is greater than the work function of Zinc, so ultraviolet can emit photo electrons even intensity is weak. While the energy of photon of red colour photon is less than work function of zinc. So the photoelectric emission is in dependent of intensity. 26. Write the Einstein's photoelectric equation in terms of the stopping potential and the threshold frequency for a given photosensitive material. Draw a plot showing the variation of stopping potential versus frequency of incident radiation. Ans. Einstein's photoelectric equation in terms of stopping potential Vs is eVs = h - 0 or Vs e - e Vs where 0 is the work function h 0 0 = frequency of incident radiation The graph of stopping potential Vs versus frequency of incident radiation is shown in Fig. Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 27. The wavelength '' of a photon and the de-Broglie wavelength of electron how the same value. Show that the energy of photon is times the kinetic energy of electron, where m, c, h have their usual 2mc meanings. h mv Ans. h E hc Ek 1 mv 2 2 1 h2 m 2 2 2 m 2 2 2hc m or mh2 E 2mc Ek h E 2mc h Ek 28. Radiations of frequency 1015 Hz are incident on two photosensitive surfaces P and Q. Following observations are made. (i) For surface P, photoelectric emission occurs but photoelectrons have zero kinetic energy. (ii) For surface Q, photoelectric emission occurs and photoelectrons have some kinetic energy. Which of there ha higher works function? If the incident frequency is slightly reduced, what will happen to the photoelectric emission in the two cases? (i) For surface P, Ek = 0, So, Energy of photon = work function h = w = h0 = 6.610-34 1015 = 6.610-19 Joule. (ii) For surface Q, the photoelectrons have some kinetic energy. h = w = Ek Work function of Q is less than that of P i.e. surface P has higher work function than Q. As the frequency of incident radiation is slightly reduced energy of photon will become, less than work function of P, but will be more Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com than the work function of Q. Hence surface P will show no photoelectric emission while Q will show photoelectric emission but the kinetic energy of photo electrons from surface Q will be lower than initial value. 29. Radiations of frequency 1015Hz are incident on two photosensitive surfaces A and B. following observations are recorded: Surface A : No photoelectric takes place. Surface B: Photoemission takes place but photoelectrons have zero energy explain the above observation on the basis of Einstein’s photoelectric equation. How will the observation with surface B change when wavelength of light is decreased? Ek = h - h0 Surface A: As no photo emission takes place; energy of incident photon is less than the work function. Surface B: As photoelectric emission takes place with zero kinetic energy of photoelectrons. i.e. energy of incident photon is equal to work function when wavelength of incident light is decreased, the energy of incident photon become more than the work function, so photoelectrons emitted will have finite kinetic energy given by. Ek he -W 30. X-rays of wavelength '' fall on a photosensitive surface, emitting electrons. Assuming that the work function of the single can be neglected. Prove that the de-Broglie wavelength of electron emitted will h be 2me h B K.E. of electrons Ek = h + w 2mE k as W = o, (work function neglected) h hc Ek = h 2m hc Ek = h B 2mc Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 31. An electromagnetic wave of wavelength is incident on a photosensitive surface of negligible. Work function. If the photoelectrons emitted from surface have the same de-Broglie wavelength B, Prove that 2mc 2 B h De-Broglie wavelength B h 2mE k h2 2mE k 2 B or h2 hc 2m 2mc 2 B h or 2mc 2 B h 32. Two lines A and B shown in the graph represent the de-Broglie wavelength () as a function of 1 v (v is the accelerating potential for two particles having the same change, which of the two represents the particle of smaller, mass. B The slope of line B is large, so particle B has smaller mass. h 2mqv h 2mq A 1 V 1 V 33. Ultraviolet light of wavelength 2271 A0 from a 100 w mercum source irradiates a photo cell made of molybdenum metal. If the stopping potential is -1.3 V, estimate the work function of the metal. How would the photo cell respond to high intensity (105 wm-2) red light of wavelength 6328 A0 produced by a He-Ne laser? (h= 6.6310-34 Js, C = 3108 m/s) Ek hc hc w Or W - E k Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com 6.6 10 34 3 10 8 - 1.6 10 19 1.3 2271 10 10 W 4.2eV hc W Or 0 0 hc 6.63 10 34 3 10 8 2977 A 0 w 6.68 10 19 As given wavelength 6328 A0 is greater than 2977 A0 the photocell will not respond to red light produced by He - Ne lese, How ever intense it may be. 34. Defined work function of a metal. the threshold frequency of a metal is f0. When the light of frequency 2f0 is incidents on the metal plate, the maximum velocity of electrons emitted is v1. When the frequency of incident radiation is increased to 5f0, the maximum velocity of electrons emitted is v2 find the ratio of v1 to v2 1 mv 2 2 In first case 2f 0 , 0 f 0 , v v1 h h 0 1 1 mv 12 mv 12 hf 0 2 2 In second case 5f 0 , 0 f 0 , v v2 h 2f 0 hf 0 1 1 mv 2 mv 2 2 2 4hf 0 2 2 (1) from eq n (2) side by side h 5f 0 hf 0 Dividing eq n - (1) - (2) v12 1 v1 1 2 v2 4 v2 2 35. Calculate the de-Broglie. Wavelength of a neutrons kinetic energy 150 eV. h 2mE k 6.63 10 -34 2 1.67 10 27 750 1.6 10 19 0.02335 A0 36. Describe Davission and Germer experiment to demonstrate he wave nature of electrons. Draw a labeled diagram of apparatus used 37. State the laws of photoelectric effect. How have they been explain by Einstein? 38.Draw a graph showing the variation of photoelectric current with anode potential of a photocell for (i) same frequency but different intensities of Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL) www.nrpschool.com incident radiation (ii) same intensity but different frequencies of incident radiation. 39. What is photoelectric effect? Explain experimentally the variation of photoelectric current with (i) intensity (ii) the p.d. between the plates (iii) frequency of incident light and hence obtain Einstein‘s photoelectric equation. 40. Calculate the maximum kinetic energy of electros emitted from a photosensitive surface of work function3.2eV, for the incident radiation of wavelength of 300nm 41.The work function of three elements a, B, and C are 5eV,3.8eV and 2.8eV respectively. A radiation of wavelength 4125 A0 is made to be incident on each of these elements. Show by appropriate calculation in which case photoelectrons will not be emitted. 42. A source of light is placed at a distance of 0.50m from photocell used and the cut of potential is found to be V0. If the distance between the light source and the photocell is made 0.25m. What will be the new cut of potential? 43.In Davission and germer experiment, state the observation s which led to (i) show the weave nature of electrons and (ii) confirm de Broglie relation. 44. Deduce de Broglie wavelength of electrons accelerated by a potential of v volt. Draw a schematic diagram of a localized wave describing the wave nature of moving electron. 10. Following table gives the values of work function for few photosensitive metals. S,No. Metal Work Function (eV) 1 Na 1.92 2 K 2.15 3 Mo 4.17 If each of these metal is exposed to radiations of wavelength 300nm, which of them will not emit photoelectrons and why? Prepared by: Abhishek Yadav (NEW RAINBOW PUBLIC SCHOOL)









