COMPOSITION OF A HYDRATE LAB Background A hydrate is an
advertisement

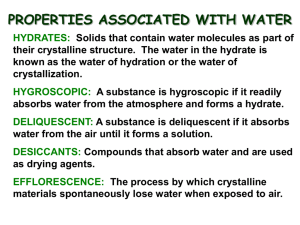
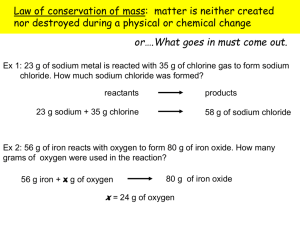
COMPOSITION OF A HYDRATE LAB Background A hydrate is an ionic compound that has a specific amount of water as part of its structure. The water is loosely bonded to the compound. Different hydrated ionic compounds will have different amounts of water that normally attach, but the number of water molecules is specific for each given hydrate. In this lab, the hydrate you will be investigating is copper(II) sulfate pentahydrate: CuSO4 · 5H2O. When the compound is dehydrated, the anhydrous salt is the remaining ionic compound. Your task is to devise a procedure in which you are able to calculate the percentage of water by mass of the copper(II) sulfate pentahydrate. Then you will determine the percent error of your measurements. Checklist □ Write a procedure for determining the percentage of water in the given hydrate. All groups will begin the lab with 3.00 g of CuSO4 · 5H2O. Any heating done should be gentle, not vigorous. After the anhydrous salt is separated and cooled, add 3 drops of water to it and record your observations. Be sure to include any specific directions for ensuring the most accurate data. Only one trial will be completed. □ Create a data table to be filled out during the lab. □ Create a list of lab equipment that will be used in the experiment. □ Create a list of safety procedures to be followed int his lab experiment. □ Have procedure approved by the teacher. □ Perform lab experiment. Record data and observations. □ Individually, write/type a lab report which includes the following headings/sections. Title Introduction paragraph (Should state the purpose of the lab, describe percent composition by mass, and differentiate between a hydrated compound and an anhydrous salt. Do not describe the procedure or results in this section.) Procedure and lab equipment used Data Table Analysis Questions: (Number and answer the questions listed on the back of this sheet) Conclusion: (Summarize the results. Discuss possible sources of error. Discuss how potential errors may have altered your data.) Analysis Questions 1) Calculate the percentage of water by mass in copper(II) sulfate pentahydrate. 2) In your procedure, how did you know all of the water was driven off the hydrate? 3) Use a percent error calculation to compare your experimental value with the theoretical value for % of water in the given hydrate. 4) How many grams of water were driven off of your compound? Show calculations. 5) How many grams of water are in 15.00 g of CuSO4 · 5H2O? Show calculations. 6) Why did the copper(II)sulfate pentahydrate anhydrous salt, which is white, turn blue again when you dripped some water into it at the end of this lab experiment? (May have to research how copper atoms behave in a solution) 7) Calculate the percent composition of water by mass in barium hydroxide octahydrate. 8) Calculate the percent composition of water by mass in mercury(I) nitrate monohydrate.