1 to 4
advertisement
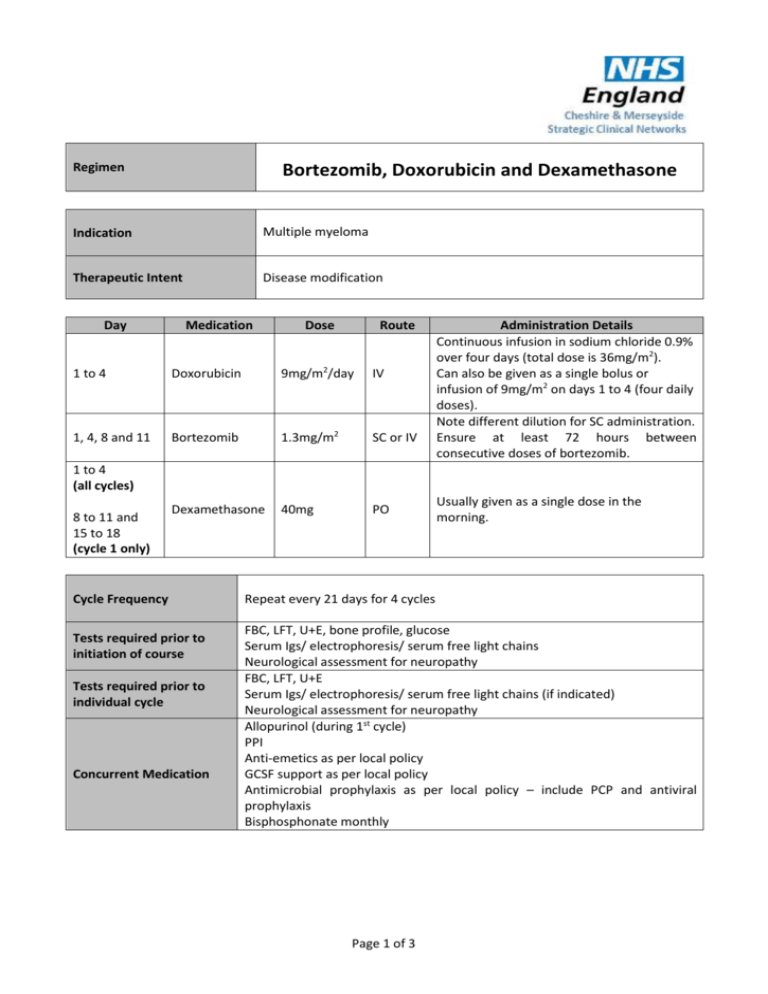
Bortezomib, Doxorubicin and Dexamethasone Regimen Indication Multiple myeloma Therapeutic Intent Disease modification Day Medication Dose Route 1 to 4 Doxorubicin 9mg/m2/day IV 1, 4, 8 and 11 Bortezomib 1.3mg/m2 SC or IV Dexamethasone 40mg PO Administration Details Continuous infusion in sodium chloride 0.9% over four days (total dose is 36mg/m2). Can also be given as a single bolus or infusion of 9mg/m2 on days 1 to 4 (four daily doses). Note different dilution for SC administration. Ensure at least 72 hours between consecutive doses of bortezomib. 1 to 4 (all cycles) 8 to 11 and 15 to 18 (cycle 1 only) Cycle Frequency Tests required prior to initiation of course Tests required prior to individual cycle Concurrent Medication Usually given as a single dose in the morning. Repeat every 21 days for 4 cycles FBC, LFT, U+E, bone profile, glucose Serum Igs/ electrophoresis/ serum free light chains Neurological assessment for neuropathy FBC, LFT, U+E Serum Igs/ electrophoresis/ serum free light chains (if indicated) Neurological assessment for neuropathy Allopurinol (during 1st cycle) PPI Anti-emetics as per local policy GCSF support as per local policy Antimicrobial prophylaxis as per local policy – include PCP and antiviral prophylaxis Bisphosphonate monthly Page 1 of 3 Dose Modifications Hepatic Renal Haematological Bortezomib Patients with moderate or severe hepatic impairment should be started on bortezomib at a reduced dose of 0.7 mg/m2 per injection during the first treatment cycle, and a subsequent dose escalation to 1.0 mg/m2 or further dose reduction to 0.5 mg/m2 may be considered based on patient tolerability (See SPC for further details). Doxorubicin Bilirubin (micromol/L) Modification 21 to 50 50% dose doxorubicin 51 to 85 25% dose doxorubicin >85 Omit Bortezomib The pharmacokinetics of bortezomib is not influenced in patients with mild to moderate renal impairment therefore, dose adjustments are not necessary for these patients. It is unknown if the pharmacokinetics of bortezomib are influenced in patients with severe renal impairment not undergoing dialysis. Since dialysis may reduce bortezomib concentrations, bortezomib should be administered after the dialysis procedure. The following parameters must be met on the first day of a new cycle (other than cycle one, when the inclusion for registration parameters must be met): Platelet count >75x109/L, Haemoglobin >8g/dl, (prior red blood cell transfusion or recombinant Human Erythropoietin usage is allowed), and neutrophil count >1x109/L. On any day of bortezomib administration during a cycle (other than day one of each cycle), the haematological results must be: Platelet count >30x109/L, Haemoglobin >8g/dl (prior red blood cell transfusion or recombinant Human Erythropoietin usage is allowed), and neutrophil count >0.75x109/L. In the event of a patient experiencing haematological toxicity >Grade 3 (neutrophil <1 x109/L and platelets <75 x109/L ) on day 1 of a cycle, the following dose reductions should be applied to all further cycles: Bortezomib: 1mg/m2, doxorubicin: 6mg/m2/day (ie 24mg/m2 total), dexamethasone: no reduction. If further haematological toxicity >Grade 3 (neutrophil <1 x109/L and platelets <50 x109/L) occurs on day 1, then the following dose reduction should be applied to all further cycles: Bortezomib: 0.7mg/m2, doxorubicin: 4.5mg/m2/day (ie 18mg/m2 total),, dexamethasone: no reduction. Dose interruption or discontinuation of treatment is not required for lymphopenia of any grade. The use of G-CSF and/or platelet support is permitted, at the discretion of the treating physician. Page 2 of 3 Peripheral neuropathy Severity of neuropathy Grade 1 (paraesthesia, weakness and/or loss of reflexes) with no pain or loss of function Modification None. Grade 1 with pain or Grade 2 (interfering with function but not with activities of daily living) Reduce bortezomib to 1.0 mg/m2 Grade 2 with pain or Grade 3 (interfering with activities of daily living) Withhold bortezomib treatment until symptoms of toxicity have resolved. When toxicity resolves reinitiate bortezomib treatment and reduce dose to 0.7 mg/m2 and change treatment schedule to once per week. Grade 4 (sensory neuropathy which is disabling or motor neuropathy that is life threatening or leads to paralysis) and/or severe autonomic neuropathy Discontinue bortezomib. Additional Information Consider modifying steroid doses for patients at risk of significant steroidrelated side-effects. References http://emc.medicines.org.uk Myeloma X clinical trial protocol version 7 Author Pharmacy CNG Approved & Checked by Haematology CNG (Review Date = Sept 2017) Page 3 of 3