AS A2 Biol Sampling weekend itinerary
advertisement

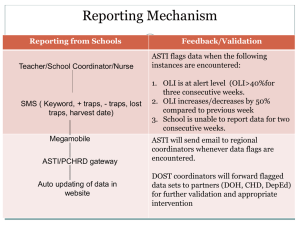
DZP AS/A2 Biology Weekend Course – Environmental Science Residential For each section we can highlight the area of the specification for the three main exam boards. Key areas covered: Population estimates Sampling techniques Running means Transects The use of keys Quadrats randomisation Practical skills: Standard error Surveying Standard deviation Microscopes Spearmans rank correlation coefficient Handling animals Mark release recapture Sampling plants and animals Activity LessonSampling plants Activity – Sampling plants Lesson – Data analysis Lunch LessonSampling animals Day 1 Summary Requirements Lesson providing background to sampling. Transects and quadrats. Raw count. Percentage cover. Numerical scalar technique. Randomisation requirements and methods Sample area of grassland using quadrats along a transect heading towards the woodland. Picnic area near big cats. Use all three methods. Collect data and add to sampling sheet. Transects from footfall path to overgrowth. Also compare percentage cover in two areas. Raw count of trees (discuss issues….what is a tree and what isn’t?) Classroom Return to classroom and analyse data collected. Statistical tests on Standard Error and Standard Deviation of the data Plenary: how does early and late succession affect biodiversity? Calculators Zoo tour introducing the different animals. At the end let students look around the zoo to count the number of wallabies? Number of deer (different species), number of Lechwe and Capybara. When students return, give real numbers, ask why are student numbers so far out when our animals are contained? What would it be like in the wild? So how do we sample animals? Jam jars, slate, pebbles Small mammal traps Muslin, elastic bands, 40mm drain pipe and fly pupae. Sheets of corrugated iron Quadrats Recording forms Tape measure 20m length of rope Random number grid Species keys Not as easy to spot? They also move? They avoid us, weather, overgrowth, remote and/or bitumen (snow leaopard, otters and Iberian wolf examples). How can we solve this problem and roofing felt. get an idea of how many animals there are in a population. Use vegetation techniques with static animals (limpets, mussels, etc) Traps; pitfall traps, small mammal traps, shrew bait tubes, tracking traps for footprints reptile and amphibian traps, Demos and discussion of radio collars, eartags, fluorescent dyes, GPS tags, camera traps Activity – Sampling animals LessonStatistical analysis preparation Set up small mammal traps. Build and set up bait tube. Build and set up hair tubes Build and set up pitfall traps. Set up footprint traps. Check reptile and amphibian traps. ID and handling of reptiles and amphibians. Prepare tables for data collection. Fit these to a chosen stats test. Can have a collection of limpet shells to measure. Compare two sites / beaches using height to width ratio and for both. Plot these on graphs and calculate the Spearmans rank of each beach, and then look at the graph to see what the actual statistical result means. Close encounters with reptiles, amphibians and invertebrates. Dinner and Set up Camp Activity – night time Get up close and personal with owls and other birds and learn how they fly and hunt and falconry display orientate at night. Download bird call apps onto mobile phones and try to communicate with local tawny owls (can we estimate population density? No. Can we estimate distribution? Yes). 1m bamboo canes, blue insulation tape. Large clear plastic bag, small weighing bags, small mammal balance / scale. Duct tape (blue) Calculators Two bags of limpet shells (one labelled sheltered, one exposed). Each limpet to have either S or E written on them. Calipers. Rulers. Guided stats sheets Activity- Sampling at night Use sugar gliders to show how similar to bats as a nocturnal “flying” mammal and to look at scorpions at night. Bat detector around the park Trying to identify animals in the park at night using infra-red and to try to establish population sizes at night (e.g wolf count). Build / set up moth traps using a variety of scented or coloured candles. Camp fire and marshmallows. Overnight camping in the park Infra red Bat detector and frequency keys Moth traps (or equipment to make them; lamps, bright bulbs, buckets, egg boxes, selection of scented or coloured candles Activity Activity – check traps Day 2 Summary Check small mammal traps – students to handle small mammals. Link to legislation such as Wildlife and Countryside Act (1981) for handling small mammals, plus CITES. Mark the small mammals Check moth trap – Use ID keys Check reptile and amphibian traps - students to handle reptiles and amphibians Students to take photos of animals found. Lesson – analysing Empty pitfall traps into trays. Use keys to identify species found and to classify them. results Small mammal population estimate using previous data from trapping on site. Use Simpson’s index to calculate biodiversity within different areas of the park (can use historical data if insufficient is collected over the weekend). Break Lesson- Mark release Mark release recapture of pond snails, or crickets, cockroaches, woodlice. recapture Calculate running means. Reliability. Activity- collect shrew Analyse shrew faeces under microscopes. tubes Identify shrew species. Use graticules to calculate the size of material observed. Use images and calculate the size of the magnification (IAM) Lunch Activity – Biological Lichen survey -Survey park using maps Indicators Relate to air quality in different areas of the park Activity-Natural sampling Owl Pellet analysis Using keys to identify species Put data from the entire weekend onto the map. “what have you found about DZP’s wildlife?” Requirements Large clear plastic bag, small weighing bags, small mammal balance / scale. Scissors. Small mammal and moth ID Keys. Cameras Calculators Trays ID keys Worksheets Pond with snails, nail varnish, pond nets, Microscopes, graticules, calculators shrew poo keys Slides etc Maps of zoo, lichen survey keys Forceps, face masks, trays, ID keys Weekend plenary Summarise areas covered: Sampling techniques. Transects Quadrats, randomisation Standard error Standard deviation Spearmans rank correlation coefficient Mark release recapture Population estimates Running means The use of keys Practical skills: Surveying Microscopes Handling animals Sampling plants and animals Note: An extra session on CITES and in situ vs ex situ conservation for Edexcel students can be provided.