etc3230-sup-0001-SupInfo-S1
advertisement
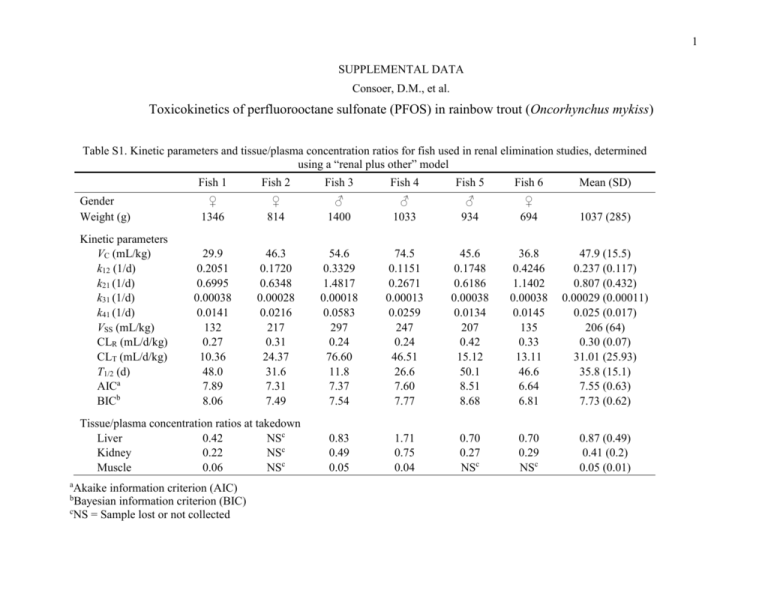
1 SUPPLEMENTAL DATA Consoer, D.M., et al. Toxicokinetics of perfluorooctane sulfonate (PFOS) in rainbow trout (Oncorhynchus mykiss) Table S1. Kinetic parameters and tissue/plasma concentration ratios for fish used in renal elimination studies, determined using a “renal plus other” model Gender Weight (g) Kinetic parameters VC (mL/kg) k12 (1/d) k21 (1/d) k31 (1/d) k41 (1/d) VSS (mL/kg) CLR (mL/d/kg) CLT (mL/d/kg) T1/2 (d) AICa BICb Fish 1 Fish 2 Fish 3 Fish 4 Fish 5 Fish 6 Mean (SD) ♀ 1346 ♀ 814 ♂ 1400 ♂ 1033 ♂ 934 ♀ 694 1037 (285) 29.9 0.2051 0.6995 0.00038 0.0141 132 0.27 10.36 48.0 7.89 8.06 46.3 0.1720 0.6348 0.00028 0.0216 217 0.31 24.37 31.6 7.31 7.49 54.6 0.3329 1.4817 0.00018 0.0583 297 0.24 76.60 11.8 7.37 7.54 74.5 0.1151 0.2671 0.00013 0.0259 247 0.24 46.51 26.6 7.60 7.77 45.6 0.1748 0.6186 0.00038 0.0134 207 0.42 15.12 50.1 8.51 8.68 36.8 0.4246 1.1402 0.00038 0.0145 135 0.33 13.11 46.6 6.64 6.81 47.9 (15.5) 0.237 (0.117) 0.807 (0.432) 0.00029 (0.00011) 0.025 (0.017) 206 (64) 0.30 (0.07) 31.01 (25.93) 35.8 (15.1) 7.55 (0.63) 7.73 (0.62) 0.83 0.49 0.05 1.71 0.75 0.04 0.70 0.27 NSc 0.70 0.29 NSc 0.87 (0.49) 0.41 (0.2) 0.05 (0.01) Tissue/plasma concentration ratios at takedown Liver 0.42 NSc Kidney 0.22 NSc Muscle 0.06 NSc a Akaike information criterion (AIC) Bayesian information criterion (BIC) c NS = Sample lost or not collected b 2 Figure S1. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 1. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. In this and Figures S2S6 measured concentrations in plasma are shown as solid dots, while open triangles denote the cumulative mass of PFOS eliminated in urine. Lines show the optimized fit of model simulations to measured values: solid line – plasma; dashed line – urine. 3 Figure S2. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 2. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. 4 Figure S3. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 3. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. 5 Figure S4. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 4. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. 6 Figure S5. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 5. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. 7 Figure S6. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 6. Simulations were obtained using a model that includes a fitted “other” elimination term k4,1. 8 Figure S7. Kinetics of PFOS elimination to expired branchial water following bolus intra-arterial injection. Data and models simulations are shown for Fish 8. In this and Figures S8-S11 measured concentrations in plasma are shown as solid dots, while open squares denote the cumulative mass of PFOS eliminated to expired water. Lines show the optimized fit of model simulations to measured values: solid line – plasma; dashed line – water. 9 Figure S8. Kinetics of PFOS elimination to expired branchial water following bolus intra-arterial injection. Data and models simulations are shown for Fish 9. 10 Figure S9. Kinetics of PFOS elimination to expired branchial water following bolus intra-arterial injection. Data and models simulations are shown for Fish 10. 11 Figure S10. Kinetics of PFOS elimination to expired branchial water following bolus intra-arterial injection. Data and models simulations are shown for Fish 11. 12 Figure S11. Kinetics of PFOS elimination to expired branchial water following bolus intra-arterial injection. Data and models simulations are shown for Fish 12. 13 Figure S12. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 2. Simulations were generated by adopting the average branchial elimination rate constant from branchial elimination studies as the elimination rate constant k4,1. In this and Figures S13-S16 measured concentrations in plasma are shown as solid dots, while open triangles denote the cumulative mass of PFOS eliminated in urine. Lines show the optimized fit of model simulations to measured values: solid line – plasma; dashed line – urine. 14 Figure S13. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 3. Simulations were generated by adopting the average branchial elimination rate constant from branchial elimination studies as the elimination rate constant k4,1. 15 Figure S14. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 4. Simulations were generated by adopting the average branchial elimination rate constant from branchial elimination studies as the elimination rate constant k4,1. 16 Figure S15. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 5. Simulations were generated by adopting the average branchial elimination rate constant from branchial elimination studies as the elimination rate constant k4,1. 17 Figure S16. Kinetics of PFOS elimination to urine following a bolus intra-arterial injection. Data and model simulations are shown for Fish 6. Simulations were generated by adopting the average branchial elimination rate constant from branchial elimination studies as the elimination rate constant k4,1. 18 Figure S17. Kinetics of PFOS in trout plasma during a continuous waterborne exposure. Data and model simulations are shown for Fish 14. In this and Figures S18-S21 measured values are shown as individual points. The fitted model simulation is shown as a solid line. 19 Figure S18. Kinetics of PFOS in trout plasma during a continuous waterborne exposure. Data and model simulations are shown for Fish 15. 20 Figure S19. Kinetics of PFOS in trout plasma during a continuous waterborne exposure. Data and model simulations are shown for Fish 16. 21 Figure S20. Kinetics of PFOS in trout plasma during a continuous waterborne exposure. Data and model simulations are shown for Fish 17. 22 Figure S21. Kinetics of PFOS in trout plasma during a continuous waterborne exposure. Data and model simulations are shown for Fish 18.








