DOCX file
advertisement
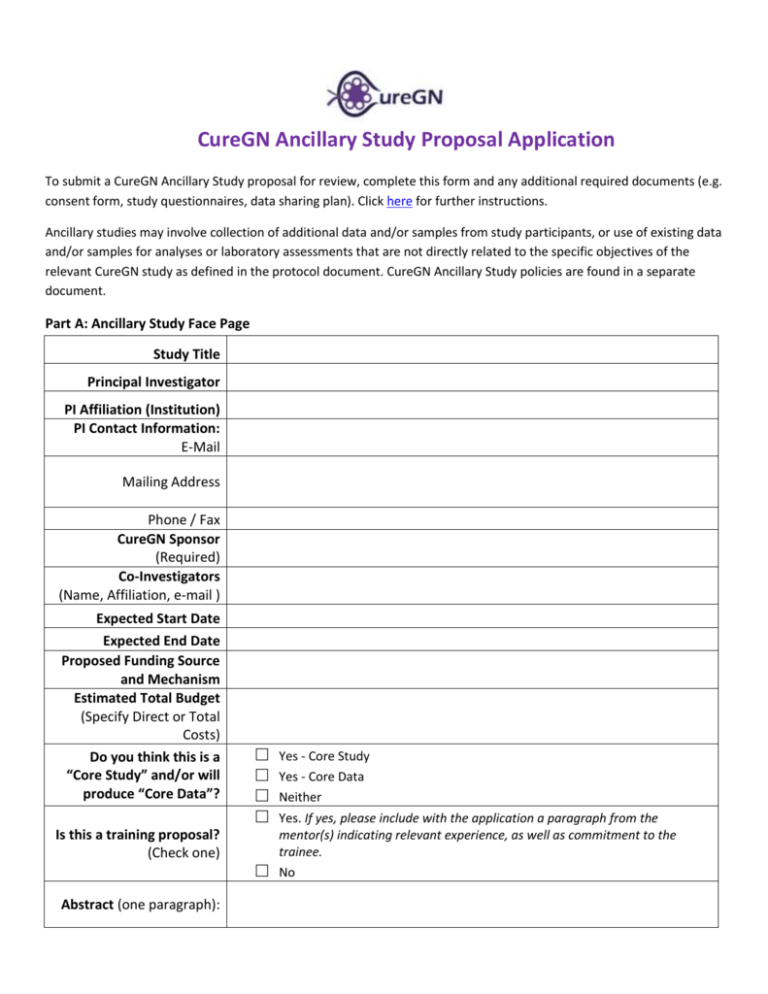
CureGN Ancillary Study Proposal Application To submit a CureGN Ancillary Study proposal for review, complete this form and any additional required documents (e.g. consent form, study questionnaires, data sharing plan). Click here for further instructions. Ancillary studies may involve collection of additional data and/or samples from study participants, or use of existing data and/or samples for analyses or laboratory assessments that are not directly related to the specific objectives of the relevant CureGN study as defined in the protocol document. CureGN Ancillary Study policies are found in a separate document. Part A: Ancillary Study Face Page Study Title Principal Investigator PI Affiliation (Institution) PI Contact Information: E-Mail Mailing Address Phone / Fax CureGN Sponsor (Required) Co-Investigators (Name, Affiliation, e-mail ) Expected Start Date Expected End Date Proposed Funding Source and Mechanism Estimated Total Budget (Specify Direct or Total Costs) Do you think this is a “Core Study” and/or will produce “Core Data”? Is this a training proposal? (Check one) Abstract (one paragraph): □ □ □ □ □ Yes - Core Study Yes - Core Data Neither Yes. If yes, please include with the application a paragraph from the mentor(s) indicating relevant experience, as well as commitment to the trainee. No Part B: CureGN Study Impact Please use this section to fully describe the impact of the ancillary study proposal on the CureGN study subjects, biospecimens, Data Coordinating Center (DCC) and consortium. Insert additional lines as necessary to fully answer the questions. B1. Requirements □ All CureGN participants OR □ B1a. □ □ □ □ □ □ Study Population Study Resources B1b. (check all that apply) Select Population (check all that apply): □ □ □ □ □ □ □ Adult Pediatrics FSGS Cohort MCD Cohort MN Cohort IGA Cohort □ □ □ Incident (first kidney biopsy within 6 months of enrollment) Incident (first kidney biopsy at a CureGN site after 1/1/2015) Prevalent Other: ______________ Use existing clinical data Use existing procured samples Use existing patient-reported data Requires new clinical data Requires additional sample procurement Requires new patient-reported data B2. Burden to Participants B2a. Does this ancillary study require additional study visits? B2b. Does this ancillary study require additional study procedures? □ □ □ □ Yes No Yes No If yes, use the table below to specify the proposed visit schedule and procedures (e.g. questionnaires, intervention, and tests) to be performed. Provide a subject level time estimate of additional study procedures (e.g. self-administered questionnaire x 10 minutes). B2c. B2d. Procedure Visit* (Time) Procedure 1 <SPECIFY> Procedure 2 <SPECIFY> Procedure 3 <SPECIFY> * Specify CureGN Visit # or additional visit time Add rows as necessary Visit* (Time) Visit* (Time) Does this ancillary study add new questionnaires/questions? If yes, provide a copy of each proposed questionnaire/question with application. □ □ Yes No If yes, check all that apply: B2e. □ □ □ □ Patient surveys Clinical data (may be collected by patient interview) Clinical data (chart review only) Other (specify): _____________________________ B3. Biosamples B3a. Use of existing specimens. Does this ancillary study require use of biospecimens collected in the core CureGN protocol? □ □ Yes No If yes, check box and provide volume for stored blood, urine, DNA, RNA samples requested Please respect the limited nature of all biorepository specimens. Individual requests exceeding 10% of original stored volume may be rejected. See https://curegn.org/publicdocuments/Biospecimens%20List.pdf for more information. Volume or Amount Type of Specimen Requested Visit(s) Whole blood for genomic DNA - EDTA tube DNA Extracted genomic DNA Whole blood for genomic RNA - PAXGene tube RNA Extracted genomic RNA B3b. EBV immortalized cell lines (PEDS ONLY) Plasma - EDTA tube Plasma - Sodium Citrate Tube Plasma - Sodium Heparin (light protected) Serum - Serum separator tube Spot urine: supernatant and cell pellet harvested at site: Spot urine cleared supernatant - with Sodium Azide (NaN3) Spot urine cleared supernatant - with protease inhibitor (PI) Spot urine cell pellet - Harvested in RNAlater stablizing Nucleic acids at RT 24-hour urine - 24-hr urine collection container B3c. □ □ New specimens: Does this ancillary study require additional specimen acquisition beyond the core CureGN protocol? If no, skip to B3f. Yes No If yes, please describe in table below: B3d. Specimen (Plasma, Serum, Urine, Other - specify) Sampling Times*: Specimen 1 <SPECIFY> Specimen 2 <SPECIFY> Specimen 3 <SPECIFY> * Specify CureGN Visit # or additional visit time Add rows as necessary Assay Proposed Volume Requested Will there be remaining biomaterials available for future use? If yes, where will B3e. they be stored and what is the expected remaining volume? □ □ Yes No B3f. Describe how testing of biologic samples (whether from stored CureGN biosamples or de novo sampling) will be performed. B3g. Will CureGN samples need to be shipped? □ □ Yes No B4. Data What existing Core data (provided as Standard Analysis Files (SAFs) are needed: (check all that apply) B4a. □ □ □ □ □ □ □ □ Demographics Comorbidities Family History Birth History Pregnancy History Prior Disease Course Medications Laboratory values/Clinical Information □ □ □ □ □ Hospitalizations and ER Visits Limited Physical Exam Patient-reported data o Quality of life o Adherence Access to DPR Imaging Other (specify: ________________) B4b. Provide schedule for Standard Analysis File (SAF) delivery and updates. (e.g., 2017 with 2 annual updates) B4c. Will this ancillary study require additional derived variables? If yes, list variables requested. □ □ Yes No If this ancillary study will result in new data, how will data be transferred to CureGN following the B4d. completion of your study? Note: genomic data will also need to be submitted to dbGAP. B4e. Describe your data sharing plan here B5. PCC Burden Describe any additional burden to PCCs or study sites. (e.g., study coordinator training, interface with B5a. participants, sample kit creation and delivery or shipping, etc.) B6. DCC Burden Will the ancillary study require the DCC to perform study monitoring activities? □ Yes B6a. (e.g., study coordinator training, IRB and/or other regulatory assistance, label □ No creation and delivery, etc.) Will any additional effort or personnel time be expected of the CureGN DCC? (Check all that apply.) B6b. If yes, describe in detail with timeline for proposed activities. □ CureGNLink questionnaire changes □ CureGN Link functionality changes □ Custom SAF □ Other (describe) Please consult the CureGN DCC early in the phase of development. Contact CureGN-ASC@arborresearch.org with questions or to discuss your study idea. Note that the proposed study may require additional activities identified during the review process. Where will the data analyses be conducted? B6c. □ □ □ The DCC will conduct all analyses, OR The ancillary study investigator (ASI) team will design and conduct the analysis with quality review from the DCC, OR The DCC will conduct some analyses, and the ancillary study investigator (ASI) team will conduct others. Please explain. B6d. For analyses conducted by DCC, will the resulting manuscript(s) be submitted by the DCC or other institution? B6e. Estimated Number & Timing of Publications (e.g. # per project year) B7 B8 □ DCC Other (name: ______) _____________ Describe your plan for obtaining IRB approval for this study. (Required prior to sample shipment/data transfer or initiation of ancillary work. Do you anticipate need for an amendment to the approved CureGN IRB package at sites where subjects are participating? (This would apply to new surveys, new biospecimens, etc.). □ □ Yes No Indicate if the study has an active Investigational New Drug (IND) or Investigational Device Exemption (IDE) number: Number B9 Responsible Investigator(s) or Sponsor(s) IND IDE B10 Does this research have the potential to be used when applying for an IND or IDE to the FDA? □ □ Yes No Part C: Study Design (3 Pages Maximum Excluding References) 1. Background and Rationale: 2. Hypothesis, Objectives and Specific Aims: 3. Study Design and Procedures: (Summary) 4. Statistical Analysis: 5. Sample Size and Justification: (Including Power Analysis) 6. Anticipated Results and Project Timeline: 7. References: (15 Maximum) Please submit completed Ancillary Study Proposal Applications with all supporting documents including NIHstyle biosketches of all PIs, as a single PDF document to CureGN-ASC@arborresearch.org.










