Answers
advertisement
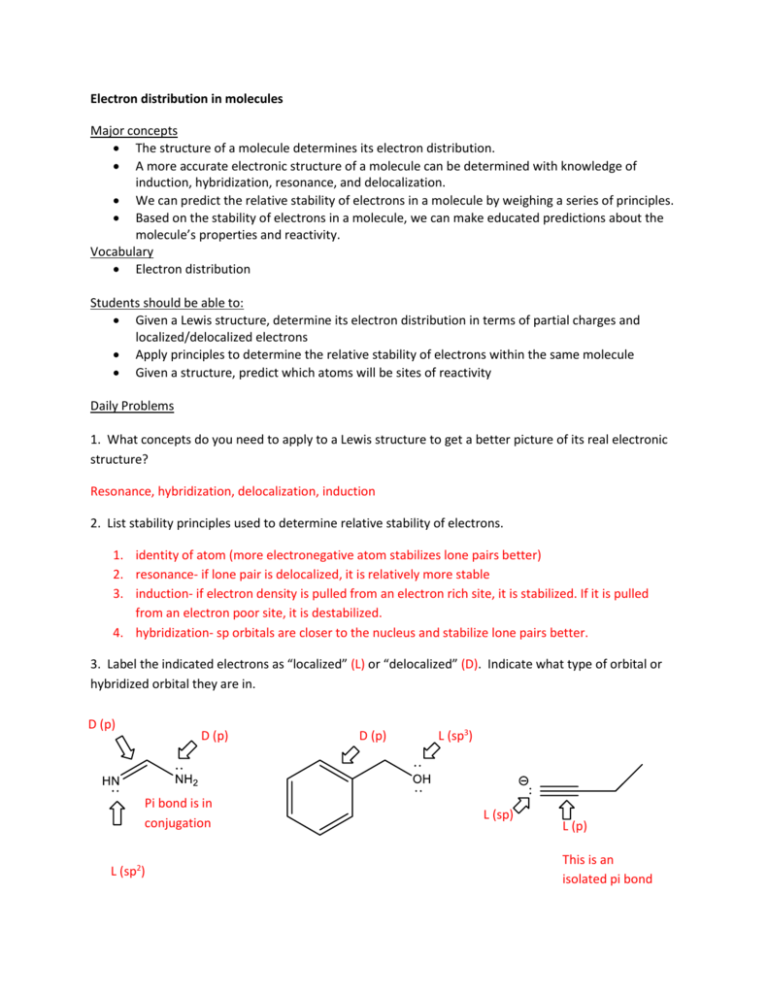
Electron distribution in molecules Major concepts The structure of a molecule determines its electron distribution. A more accurate electronic structure of a molecule can be determined with knowledge of induction, hybridization, resonance, and delocalization. We can predict the relative stability of electrons in a molecule by weighing a series of principles. Based on the stability of electrons in a molecule, we can make educated predictions about the molecule’s properties and reactivity. Vocabulary Electron distribution Students should be able to: Given a Lewis structure, determine its electron distribution in terms of partial charges and localized/delocalized electrons Apply principles to determine the relative stability of electrons within the same molecule Given a structure, predict which atoms will be sites of reactivity Daily Problems 1. What concepts do you need to apply to a Lewis structure to get a better picture of its real electronic structure? Resonance, hybridization, delocalization, induction 2. List stability principles used to determine relative stability of electrons. 1. identity of atom (more electronegative atom stabilizes lone pairs better) 2. resonance- if lone pair is delocalized, it is relatively more stable 3. induction- if electron density is pulled from an electron rich site, it is stabilized. If it is pulled from an electron poor site, it is destabilized. 4. hybridization- sp orbitals are closer to the nucleus and stabilize lone pairs better. 3. Label the indicated electrons as “localized” (L) or “delocalized” (D). Indicate what type of orbital or hybridized orbital they are in. D (p) D (p) Pi bond is in conjugation L (sp2) D (p) L (sp3) L (sp) L (p) This is an isolated pi bond Cumulative problems Most problems in this section are cumulative. All that we have learned up to this point is for the purpose of getting a relatively accurate electron distribution picture of a molecule. 4. Which atom(s) hold the most reactive lone pair in these molecules? Explain your process. Which principle(s) did you use? NH ₂ NH ₂ N N difference in hybridization sp³ is less stable and more reactive O localized O H N OH O charged lone pairs are more reactive based on resonance the lone pairs are delocalized over two oxygens nitrogen is the less electronegative atom, so the lone pair is less stabilized Due to resonance, all three of these atoms "hold" the lone pair 5. Label these atoms as “Lewis acid”(LA), “Lewis base”(LB), or “Neither”(N). Be sure to consider the real structure. LA LB LB LB LB LA LA LA N LB N LB LA LB N N LA N LA 6. Which of these compounds is a more reactive Lewis acid? b a The most reactive is a. Both have partial positive charges on the carbonyl, but the amide stabilizes this charge The more reactive is c. Compound c has a resonance stabilized (less reactive) carbocation. d c The more reactive is f because it has a greater partial positive charge due to induction. e f 7. Which ion is more stable? Which is less reactive? The first compound is more stable and less reactive than the second. Both are charged and delocalized, but the difference is the identity of the atom. Oxygen (electronegative) is a better stabilizer. O The first compound is more stable and less reactive due to resonance stabilization. Likewise, the second compound is destabilized by resonance that puts a positive charge next to a partial positive charge. Extension problems We will take a break from extension problems—Go study for exam 1!