Number of moles of copper
advertisement

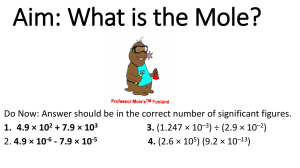
S 4 chemistry / revision exercise / mole concept / 03-04 / P.1 Mole concept 1. Find the number of moles of ions in (a) 2 moles of Fe2(SO4) 3 From the formula, we know that 1 mole of Fe2(SO4) 3 contains 2 moles of Fe3 ions and 3 moles of SO42 ions. = = = 22=4 32=6 4 + 6 = 10 = = = 0.2 0.2 3 = 0.6 0.2 + 0.6 = 0.8 No. of moles of Fe3 ions No. of moles of SO42 ions Total no. of moles of ions (b) 0.2 moles of Al(NO3) 3 2. No. of moles of Al3 ions No. of moles of NO3 ions Total no. of moles of ions Given 1.6 g of methane (CH4), find (a) number of moles of CH4 = 12 + 1 4 = 16 gmol1 = mass of CH4 /molar mass of CH4 = 1.6/16 = 0.10 (b) number of molecules of CH4 Molar mass of CH4 No. of moles of CH4 No. of molecules of CH4 = = = no. of moles Avogadro Number 0.10 6.02 1023 6.02 1022 (c) number of H atoms. One CH4 molecule contain 4 H atoms, 3. 4. no. of H atoms = = 6.02 1022 4 2.41 1023 Find the mass of (a) 1 H2O molecule 18 / 6.02 1023 3.0 1023 g (b) 1 Cu atom 63.5 / 6.02 1023 1.05 1022 g (c) 1 Na ion 23 / 6.02 1023 3.8 1023 g (d) 1 OH ion 17 / 6.02 1023 2.8 1023 g (e) 1 neutron 1.0 / 6.02 1023 1.7 1024 g (f) 1 electron 0.00055 / 6.02 1023 9.1 1028 g Find the mass of 6.02 1022 lead atoms 3.01 1024 carbon dioxide molecules No. of moles of Pb atoms No. of moles of CO2 = 6.02 1022 / 6.02 1023 molecules = 0.100 = 3.01 1024 / 6.02 1023 = 5.00 Mass of Pb atoms Molar mass of CO2 = 0.100 207 = 12 + 16 2 = 44 g = 20.7 g Mass of CO2 molecules = 5.00 44 = 220 g 3.01 1023 sulphate ions No. of moles of SO42 ions = 3.01 1023 / 6.02 1023 = 0.500 Molar mass of SO42 = 32 + 16 4 = 96 g Mass of SO42 ions = 0.500 96 = 48 g S 4 chemistry / revision exercise / mole concept / 03-04 / P.2 5. (a) How many molecules are there in 3.00 moles of oxygen molecules? Number of oxygen molecules = = 3.00 6.02 1023 1.806 1024 (b) How many ions are there in 0.600 moles of potassium ions? Number of potassium ions = = 6. 0.600 6.02 1023 3.612 1023 (Relative atomic masses: O = 16.0, K = 39.0) Calculate the number of moles of atoms in (a) 127 g of copper Number of moles of copper = 127 / 63.5 = 2 mol (b) 12.8 g of sulphur. Number of moles of sulphur = 12.8 / 32.0 = 0.4 mol (Relative atomic masses: Cu = 63.5, S = 32.0) 7. How many atoms are there in (a) 2.50 moles of oxygen atoms? Number of oxygen atoms = = 2.50 6.02 1023 1.505 1024 (b) 6.00 g of magnesium atoms? Number of mole of magnesium atoms = 6.00 / 24.0 = 0.25 mol Number of magnesium atoms 8. = 0.25 6.02 1023 = 1.505 1023 What is the mass of 2.50 moles of magnesium atoms? (Relative atomic mass: Mg = 24.0) Mass of magnesium atoms = = 2.50 24.0 60.0 g S 4 chemistry / revision exercise / mole concept / 03-04 / P.3 9. A pure sample of calcium chloride CaCl2 was found to contain 7.10 g of Cl ions. What mass of Ca ions does the sample contain? Number of moles of Cl ions= = 7.10 / 35.5 0.200 The formula CaCl2 shows that the ratio of Ca2 ions to Cl ions is 1 : 2, hence no. of moles of Ca2 ions = 0.200 / 2 = 0.100 Mass of Ca2 ions in the sample = 0.100 40 = 4.0 g 10. A metal M ionizes to give Mn ions. If atomic mass of M is 24, and 1.2 g of M ionize to give 6.02 1022 electrons, calculate n (the charge on each ion of M). No. of moles of electrons given = no. of electrons / Avogadro Number = 6.02 1022 / 6.02 1023 = 0.100 No. of moles of M ionized = 1.2 / 24 = 0.050 0.050 mole of M gives 0.100 mole of electrons on ionization. Thus 1 mole of M gives 0.100 / 0.050 = 2 moles of electrons. Since the charge on an ion of M is numerically equal to the number of moles of electrons given by 1 mole of M, each ion of M carries 2 charges, i.e. n = 2. 11. Complete the table below: Substance Sulphuric acid (H2SO4) Molar mass of substance Mass of substance present Number of moles of substance present Number of molecules/ formula units present 98 g mol1 58.8 g 0.6 mol 3.612 1023 Sodium hydroxide 2.0 g 0.05 mol 40 g mol1 3.01 1022 (NaOH) Potassium carbonate 2.4 mol 331.2 g 138 g mol1 1.44 1024 (K2CO3) (Relative atomic masses: C = 12.0, H = 1.0, K = 39.0, Na = 23.0, O = 16.0, S = 32.0) 12. If we breathed in 3913 1018 molecules of air pollutant, nitrogen dioxide (NO2), how many grams of NO2 would we breathe in? (Relative atomic masses: N = 14.0, O = 16.0) Number of mole of nitrogen dioxide = 3913 1018 / 6.02 1023 = 6.5 103 mol Mass of nitrogen dioxide = = 13. (I) 6.5 103 (14.0 + 2 16.0) 0.299 g How many moles of calcium fluoride (CaF2) are present in 16.5 g of it? Formula mass of CaF2 = 40.0 + 19.0 2 = 78.0 g Number of mole of CaF2 = 16.5 / 78.0 = 0.21 mol S 4 chemistry / revision exercise / mole concept / 03-04 / P.4 (II) How many calcium and fluoride ions are present? Number of Ca2+ ions = 0.21 6.02 1023 = 1.26 1023 Number of F ions = 0.42 6.02 1023 = 2.53 1023 (Relative atomic masses: Ca = 40.0, F = 19.0) 14. What mass of water contains the same number of molecules as 2.20 g of carbon dioxide? (Relative atomic masses: C = 12.0, H = 1.0, O = 16.0) Number of mole of CO2 = 2.20 / (12.0 + 2 16.0) = 0.05 mol Mass of water = = 0.05 (1.0 2 + 16.0) 0.9 g 15. Identity the substance which contains the greater number of molecules from each set: (a) 2 moles of carbon dioxide molecules (CO2) or 8.40 g of sulphuric acid (H2SO4) Number of CO2 molecules = 2 6.02 1023 = 1.20 1024 (Greater number of molecules) Number of mole of H2SO4 = 8.40 / (1.0 2 + 32.0 + 16.0 4) = 0.086 mol Number of H2SO4 molecules = 0.086 6.02 1023 = 5.16 1022 (b) 88.0 g 0f carbon dioxide (CO2) or 84.0 g of nitrogen (N2) Number of CO2 molecules = (88.0 / 44.0) 6.02 1023 = 1.20 1024 Number of mole of N2 = = (84.0 / 28.0) 6.02 1023 1.81 1024 (Greater number of molecules) (c) 5 1024 ammonia molecules (NH3) or 8.00 g of sulphuric acid (H2SO4) Number of NH3 molecules = 5 1024 (Greater number of molecules) Number of mole of H2SO4 molecules = = [8.00 / (1.0 2 + 32.0 + 16.0 4)] 6.02 1023 4.91 1022 (Relative atomic masses: C = 12.0, H = 1.0, N = 14.0, O = 16.0, S = 32.0) S 4 chemistry / revision exercise / mole concept / 03-04 / P.5 16. Calculate the molarity of each of the following solutions: (a) 23.4 g of sodium chloride (NaCl) solid in 1.0 dm3 solution. Number of mole of NaCl = 23.4 / (23.0 + 35.5) = 0.4 mol Molarity of NaCl solution = 0.4 / 1 = 0.4 M (b) 17.0 g of silver nitrate (AgNO3) solid in 200.0 cm3 solution. Number of mole of AgNO3 = 17.0 / (108.0 + 14.0 + 16.0 3) = 0.1 mol Molarity of AgNO3 solution = 0.1 / (200.0 / 1000.0) = 0.5 M (Relative atomic masses: Ag = 108.0, Cl = 35.5, N = 14.0, Na = 23.0, O = 16.0) 17. Complete the table below: Substance KOH CuSO4 (COOH) 2˙2H2O Molar mass Concentration (g mol-1) (g dm-3) Molarity (M) Mass of solute required to prepare 250.0 cm3 of solution (g) 5.60 g dm-3 × (250.0/1000) dm3 = 1.40 g 56.0 g mol-1 5.60 g dm-3 0.10 M 159.5 g mol-1 31.9 g dm3 0.20 M 7.975 g 126.0 g mol-1 30.24 g dm3 0.24 M 7.56 g (Relative atomic masses: C = 12.0, Cu = 63.5, H = 1.0, N = 14.0, O = 16.0, S = 32.0) 18. Calculate the molarity of each of the following solutions (a) 500.0 cm3 of 0.10 M sodium hydrogencarbonate (NaHCO3) solution. Number of mole of NaHCO3 = 0.10 (500.0 / 1000.0) = Mass of NaHCO3 0.05 mol = 0.05 (23.0 + 1.0 + 12.0 + 16.0 3) = 0.05 84 = 4.2 g (b) 100.0 cm3 of 3.00 M potassium dichromate (K2Cr2O7) solution. Number of mole of K2Cr2O7 = 3.00 (100.0 / 1000.0) = Mass of K2Cr2O7 0.3 mol = 0.3 (39.0 2 + 52.0 2 + 16.0 7) = 0.3 294 = 88.2 g (Relative atomic masses: C = 12.0, Cr = 52.0, H = 1.0, K = 39.0, Na = 23.0, O = 16.0) S 4 chemistry / revision exercise / mole concept / 03-04 / P.6 19. 5.6 g of a metal M combine with 2.4 g of oxygen to form an oxide with the formula M2O3. What is the atomic mass of M? Let the atomic mass of M be Ar. Mass of M in compound / Mass of oxygen in compound = 2Ar / 16 3 = 5.6 / 2.4 = 56 Hence atomic mass of the metal M is 56. 20. A crystalline salt of formula M2S2O3 . 5H2O is found to contain 36.3% by mass of water of crystallization. Calculate (a) the formula mass of the hydrated salt (b) the atomic mass of the metal M. (a) 5(1 2 + 16) / formula mass of hydrated salt = 36.3 / 100 = 248 Formula mass of hydrated salt = 248. (b) Let the atomic mass of M be A. Formula mass of hydrated salt = 2Ar2 + 32 2 + 16 3 + 5(1 2 + 16) = 248 Ar = 23 Therefore the atomic mass of M is 23. 21. 10.0 g of hydrated iron(II) sulphate, Fe2SO4.nH2O, on strong heating, gave 4.53 g of water. Find the value of n. Relative mass (g) Relative no. of mass FeSO4 5.47 5.47 / 152 = 0.036 0.036 / 0.036 = 1 H2 O 4.53 4.53 / 18 = 0.252 0.252 / 0.036 = 7 Therefore, n = 7 22. A metal M forms two chlorides A and B which contains 55.9% and 65.5% by mass of chloride respectively. The empirical formula of A is found to be MCl2. Determine the empirical formula of B(without having to find the atomic mass of M). If mass of M in chloride B is the same as that in chloride A (i.e. 44.1 g), mass of chlorine in chloride B will be (by proportion) equal to 65.5 44.1 / 34.5 = 83.7 g Therefore for the same mass (hence same no. of moles) of M, Mass of chlorine in chloride B / Mass of chlorine in chloride A = no. of moles of chlorine in chloride B / no. of moles of chlorine in chloride A = 83.7 / 55.9 = 1.5 Since formula of chloride A is given to be MCl2, formula of chloride B should be MCl3.