Pearls in Functional Neurosurgery
advertisement

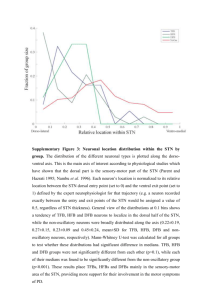
Pearls in Functional Neurosurgery Targeting the STN: MRI sequences and image fusion tips Phillip Starr, United States Our DBS group utilizes mainly T2 weighted fast spin echo (FSE) sequences for “direct” targeting of the STN. Our MRI protocol has changed little since originally described.1,2 We acquire images in an axial plane, at 2 mm slice thickness, using an “interleaved” protocol such that every other slice is acquired in the first half of the scan, followed by the second set in the last half. Interleaving is a standard technique to improve tissue contrast, which can be reduced in T2 FSE imaging if contiguous image slice images are obtained simultaneously. We prefer a 1.5T magnet for more accurate fusing of images to one another and to CT exams. We also obtain a gadolinium enhanced T-1 weighted volumetrically acquired gradient echo sequence, to use primarily for trajectory planning. For standard frame based-stereotaxy, we acquire the MRI the day prior to surgery, import into a surgical planning software package (Medtronic Framelink, or Brainlab), and plan the target and trajectory. After reformatting the images orthogonal to the axial plane containing the anterior and posterior commissures (AC-PC plane), the STN target is selected on the axial plane that best shows the medial border of the STN. This plane is typically 4 mm inferior to the AC-PC plane, and shows the dorsal red nuclei. We draw a line in the right-left axis, from the midline, tangent to the anterior border of the red nucleus, crossing he medial border of the STN and extending 2 mm into the STN from its medial border. The medial border is typically better defined on T2-FSE imaging than any other border. The planned target is also visualized on coronal reconstructions, where it is typically 6-8 mm from the edge of the cerebral peduncle at the upper midbrain. A trajectory is planned to the target from a starting point near the coronal suture, close to 3 cm from midline, with adjustments made to avoid the ventricle, sulci, and GAD-enhancing vessels. Computational fusion of the MRI to a stereotactic CT has the potential to introduce targeting error. It is very important, after fusion, to visualize the AC and PC in each image set to make sure they superimpose. Fusion errors of 0.5-1 mm at the level of the commissures are common. Techniques to minimize these fusion errors include: (1) Acquire the stereotactic CT with adequate energy transmission. CT technicians may try to minimize the energy levels of the x-rays used to reduce radiation to the patient, but the resulting tissue contrast may compromise some image fusion algorithms slightly. (2) If image fusion problems occur, consider using 1.5T MRI rather than 3T. (3) Understand the fusion errors in your particular system by visualizing the commissures carefully on all images, and try different surgical planning software packages if one vendor’s package is problematic. For stereotactic CT, placing the front pins higher than 1 cm above the eyebrow, and the back pins lower than the widest diameter of the head, will tend to avoid pin artifact at the level of the commissures and allow better visualization of the commissures on CT to confirm precise fusion. For DBS placement using interventional MRI, there are some additional considerations for image acquisition. T2 FSE sequence parameters are adjusted slightly to reduce radiofrequency energy transmission, as the iMRI technique utilizes a body transmit coil rather than a head transmit/receive coil.3 1. 2. 3. Starr PA: Placement of deep brain stimulators into the subthalamic nucleus or Globus pallidus internus: technical approach. Stereotact Funct Neurosurg 79:118-145, 2002 Starr PA, Christine C, Theodosopoulos PV, Mosely T, Byrd D, Lindsey N, et al: Implantation of deep brain stimulator electrodes into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified electrode locations. J Neurosurg 97:370-387, 2002 Starr PA, Martin AJ, Ostrem JL, Talke P, Levesque N, Larson PS: Subthalamic nucleus deep brain stimulator placement using high-field interventional magnetic resonance imaging and a skull-mounted aiming device: technique and application accuracy. J Neurosurg 112:479-490, 2010