View/Open
advertisement

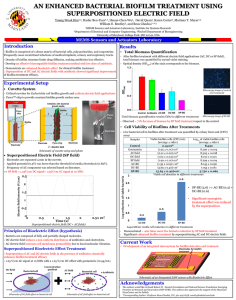
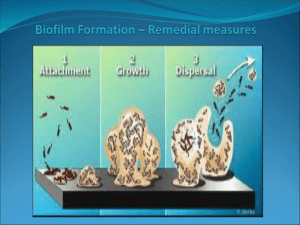
Importance of biofilm formation and dipeptidyl peptidase IV for the pathogenicity of clinical Porphyromonas gingivalis isolates For figures, tables and references we refer the reader to the original paper. Introduction Porphyromonas gingivalis is a natural member of the oral microbiome and regarded as a primary etiological agent of periodontitis (Holt et al., 1999; Davey, 2006; Hayashi et al., 2010; Cugini et al., 2013). This Gram-negative anaerobe is a key player in the shift from benign dental plaque into a pathogenic microbial community (Cugini et al., 2013). Thanks to an arsenal of specialized virulence factors, P. gingivalis can become highly destructive and trigger the progression of periodontal disease (Cugini et al., 2013). Porphyromonas gingivalis is known to have proteolytic properties and digests external sources of peptides to meet its nutritional requirements (Banbula et al., 2000; Kumagai et al., 2000; Rea et al., 2004; Cugini et al., 2013). The serine protease dipeptidyl peptidase IV (DPPIV) cleaves X-Pro or X-Ala dipeptides from the N-terminal end of polypeptide chains, leading to the breakdown of periodontal tissues (Banbula et al., 2000; Kumagai et al., 2000). Moreover, DPPIV promotes the activity of host-derived matrix metalloproteinase-1 (MMP-1), MMP-2, MMP-8 and MMP-9, which may augment tissue destruction (Kumagai et al., 2005). DPPIV also influences the host's healing process, as it inhibits the adhesion of fibroblasts to fibronectin (Kumagai et al., 2005). The ability of P. gingivalis to colonize the oral cavity and proliferate is influenced by its capacity to participate in the oral biofilm (Davey, 2006). Biofilm formation may improve the adherence to the subgingival tissue and provide protection against the immune system (Cos et al., 2010). Furthermore, the presence in a microbial community may lead to environmental conditions and interactions that are pivotal to elicit a virulent state and full pathogenicity of P. gingivalis (Cugini et al., 2013). The aim of the study is to evaluate the importance of biofilm formation and DPPIV activity for the virulence of clinical P. gingivalis isolates. Their biofilm-forming capacity and DPPIV activity were assessed in relation to their in vivo pathogenic potential. Moreover, bacterial DPPIV activity was compared between planktonic and sessile cells in biofilms to evaluate the influence of the mode of growth on DPPIV. Methods Bacterial strains and culture Clinical isolates of P. gingivalis with a different capsular type (K1–K5) were obtained from the oral cavity of patients at the University Hospital Leuven (Belgium). The ethical committee of the University Hospital approved the study (Code B32220071073) and the written informed consent was obtained from all subjects. Capsular serotyping of the clinical isolates was performed by double immunodiffusion and immunoelectrophoresis using polyclonal antisera, as described previously (van Winkelhoff et al., 1993; Laine et al., 1996; Dierickx et al., 2003). All strains were grown on Wilkins– Chalgren agar plates (Oxoid) supplemented with 5% sheep blood (Oxoid) and l-cysteine (500 mg L−1; Sigma-Aldrich). The plates were incubated at 37 °C in an anaerobic atmosphere of 80% N2, 10% H2, and 10% CO2 for 7–14 days. Biofilm determination A protocol using crystal violet for the quantification of biofilms was optimized for P. gingivalis (Tote et al., 2010). Biofilms were grown in a 96-well plate using Wilkins–Chalgren broth (Oxoid) supplemented with 500 mg L−1 l-cysteine (Sigma-Aldrich), 2 g L−1 maltose (Merck), and 2 g L−1 starch. The latter two compounds were added to improve the biofilm-forming capacity. After an incubation period of 96 h on a horizontal shaking plate (Innova 4300; New Brunswick Scientific) under anaerobic conditions, the medium was removed and the wells were washed with phosphatebuffered saline (Gibco) to remove nonadherent bacteria. The biofilm was methanol-fixed, stained with 0.01% (w/v) crystal violet (Merck), and washed with running tap water to remove unbound dye. The wells were air-dried, whereupon 33% (v/v) glacial acetic acid (Merck) was added to release the dye from the biofilms. The optical density was measured at 570 nm using a microplate reader (Multiskan microplate reader, Labsystems). P. gingivalis reference strains ATCC 33277, W50, and W83 were used as controls, as they are known for good, moderate, and weak biofilm formation, respectively (Davey, 2006; Dashper et al., 2010; Biyikoglu et al., 2012). Based on these phenotypes, three categories of biofilm formation were defined. An optical density under 0.2 was categorized as no or weak biofilm formation, an optical density between 0.2 and 0.5 was considered as moderate biofilm development and based on the reference strain ATCC 33277, an optical density higher than 0.5 was chosen as a cut-off for good biofilm formation. According to these arbitrary values, the phenotype of the clinical isolates could be defined. For each strain, at least eight replicates were taken and the experiments were repeated on three different days. The biofilm producing reference strain ATCC 33277 was included as a positive control in each experiment and noninoculated Wilkins– Chalgren broth as a blank. Measurement of DPPIV activity in planktonic and in sessile P. gingivalis cells All strains were grown anaerobically in chopped meat carbohydrate broth (BD) for 24 h. Prior to fractionation, the number of colony-forming units (CFU) was determined by viable plate count. Figure 1 shows the fractionation protocol for DPPIV that was based on Banbula et al. (2000). Briefly, the cells were washed and membrane-bound enzymes were detached from the purified bacteria with a buffer solution containing 0.05% Triton X-100. To obtain the intracellular enzymes, cells were lysed with 1% Triton X-100 buffer solution. To explore the subcellular localization of DPPIV, all fractions of the reference strain W50 were examined. The DPPIV activity was measured kinetically during 20 min using 0.5 mM of the chromogenic substrate glycyl-prolyl- para-nitroanilide in 100 mM Tris-HCl buffer pH 8.3 at 37 °C (Durinx et al., 2000; Matheeussen et al., 2012). The release of paranitroaniline was measured at 405 nm using a microplate reader (VERSAmax™ microplate reader, Molecular Devices). One unit of activity was defined as the amount of enzyme, which cleaves 1 μmol substrate min−1. The enzyme activity was then related to the number of CFU and further expressed as units (U) per 1010 CFU. The commercially available DPPIV inhibitor vildagliptin was included as a control for enzyme specificity. One-way anova indicated assay repeatability, as the analysis of DPPIV activity of W50 in triplicate was the same on five independent days. The DPPIV activity in biofilms vs. planktonic bacteria was analyzed for the P. gingivalis strains with good biofilm formation. Bacteria were grown in a 24-well plate using supplemented Wilkins– Chalgren broth during 96 h and in an anaerobic atmosphere under shaking conditions. Bacteria in their biofilm state were obtained by scraping and planktonic bacteria could be harvested from the supernatant in the same wells. The amount of bacteria was determined using viable plate count and quantitative PCR (qPCR). DNA extraction and purification were performed with a QIAamp DNA Mini Kit (Qiagen) according to the manufacturer's guidelines. Primers and probes were designed from the species-specific region on 16S rRNA gene. The sequence of the forward primer was 5′GAGGAACCTTACCCGGGAT-3′; the sequence of the reverse primer was 5′ATGCAGCACCTACATAGAAGC-3′; and the sequence of the TaqMan probe was 5′TAGATGACTGATGGTGAAAACCGTCTTCC-3′ (TIB MOLBIOL). The probe was labeled with the fluorescent dyes 6-FAM at the 5′ end and BBQ at the 3′ end. Quantification of DNA with qPCR was executed with a StepOnePlus™ RT-PCR system (Applied Biosystems). Thermal cycling consisted of an initial denaturation step of 50 °C for 2 min and 95 °C for 10 min, followed by 40 amplification cycles at 95 °C for 15 sec (denaturation) and 60 °C for 1 min (annealing + elongation). All samples were processed in triplicate. Animals Eleven-week-old female BALB/c mice (Janvier) were maintained at 20 °C, 50% humidity and at a light/dark cycle of 12/12 h. All mice were provided with standard rodent food pellets (Carfil Quality) and tap water ad libitum. All animal studies were approved by the ethical committee of the University of Antwerp (Code 2011-18). Assessment of pathogenicity in mouse abscess model The pathogenic capacity of the different P. gingivalis strains was assessed in a mouse abscess model. For each strain, four mice were infected with a dorsal subcutaneous injection of bacterial suspension (0.1 mL, 109 CFU mL−1). Prior to infection, the bacterial load was adjusted by measurement of the optical density. The exact number of bacteria in the inoculum was determined by viable plate count. Exposure to the aerobic atmosphere was limited prior to infection to ensure bacterial viability. General health and weight of the mice and the presence and location of abscesses were evaluated daily. Ten days after infection, the animals were euthanized by cervical dislocation. A macroscopic evaluation was performed and the spread of P. gingivalis from the lesions to distant places was assessed by two independent experienced researchers. Statistical analysis All data were analyzed using spss software version 20.0 (SPSS, Chicago). The repeatability of the enzyme activity measurements, the localization of DPPIV, and the differences in biofilm formation and DPPIV activity between the strains were analyzed using one-way anova and Tukey's HSD post hoc tests. All values were expressed as the mean ± SD. A P-value lower than 0.05 was considered as statistically significant. Results Clinical isolates K4 and K5 demonstrated the strongest biofilm formation To evaluate the in vitro biofilm formation of the clinical isolates, P. gingivalis reference strains ATCC 33277, W50, and W83 were used as controls. Based on these phenotypes, three categories of biofilm formation were defined with good, moderate, and weak biofilm formation, respectively (Fig. 2). According to these criteria, the clinical isolates K1 and K2 formed weak biofilms, whereas K3 demonstrated moderate biofilm formation. The isolates K4 and K5 showed good biofilm development, just as the ATCC reference strain. Clinical isolates K4 and K5 showed the highest DPPIV activity in planktonic state Our fractionation protocol allowed us to examine the DPPIV activity of the P. gingivalis reference strains and clinical isolates. Fraction 2 contained detached membrane-associated proteins and showed 100 times more DPPIV activity compared to the intracellular fraction 3. This confirms the membrane association of DPPIV (Table 1) (Banbula et al., 2000). Moreover, addition of the DPPIV inhibitor vildagliptin (20 μM) to fraction 2 resulted in complete inhibition of enzyme activity. Figure 3 illustrates the DPPIV activity for all the P. gingivalis strains in planktonic state. The clinical isolates K4 and K5 demonstrated the strongest DPPIV activity, as compared to the other strains. The DPPIV activity differs significantly between the membrane-associated protein fraction (fraction 2) and the intracellular fraction (fraction 3). Fraction 1, containing whole bacteria, showed similar DPPIV activities as the membrane-associated protein fraction. ( n = 5, P < 0.05, one-way anova and Tukey's HSD post hoc test). Biofilm sessile cells show increased DPPIV activity compared to planktonic cells For the biofilm-forming clinical isolates, the DPPIV activity was compared between planktonic cells and sessile cells in a biofilm. The biofilm-associated bacteria demonstrated significantly increased DPPIV activities compared to the free-floating planktonic cells (Fig. 4). Clinical isolates K4 and K5 induce abscess formation in vivo A murine abscess model was used to study the pathogenicity of clinical isolates in vivo. The virulent reference strains W50 and W83 induced abscesses in only one out of four mice. In contrast, infection with the clinical isolates K4 and K5 resulted in clear abscess formation. In case of K4, one open and two closed abscesses were seen. Infection with clinical isolate K5 resulted in closed abscess formation in three of four mice. Two mice infected with isolate K5 even had an abdominal wound, indicating the bacterial spread from the abscess point to distant places. Viable plate counts of the pus allowed to quantify the bacterial load and confirmed the uniformity of the recovered P. gingivalis population based on morphological characteristics (data not shown). No abscess formation was seen for the reference strain ATCC 33277, or for the clinical isolates K1, K2 and K3. Discussion Dental plaque is an oral biofilm of bacteria that may induce periodontitis. The onset of periodontal disease is characterized by the shift of a health-associated biofilm of Gram-positive aerobes into a more pathogenic community with Gram-negative anaerobes (Holt et al., 1999; Cugini et al., 2013). P. gingivalis has been considered as a key player in this dysbiosis (Cugini et al., 2013) and possesses an arsenal of virulence factors for initiation and progression of periodontitis (Davey, 2006). In the oral biofilm, P. gingivalis deploys fimbriae, adhesins and a capsule to efficiently colonize the oral cavity (Davey, 2006). Because proteins are scarce and difficult to obtain in dental plaque, a variety of bacterial proteases are required to support the asaccharolytic growth of P. gingivalis. Their enzymatic activities may lead to destruction of periodontal tissue and affect the immune system (Cugini et al., 2013). Furthermore, extensive proteolysis alters the oral microenvironment and triggers the shift in biofilm composition (Cugini et al., 2013). Disruption of the bacterial interactions and disturbance of the complex interplay between the microbial community and its host form the basis of periodontal disease progression (Cugini et al., 2013). Given the central role of P. gingivalis in the development of periodontitis, profound understanding of its virulence factors and pathogenic properties may provide new insights in the pathogenesis and clues for novel therapeutic approaches. This study assessed the biofilm-forming capacity and DPPIV activity of clinical P. gingivalis isolates in relation to their in vivo pathogenic potential. Moreover, the bacterial DPPIV activity was investigated according to its mode of growth to evaluate whether P. gingivalis biofilm formation influences the regulation of other virulence factors. In our study population, bacteria with good biofilm-forming properties demonstrated a high DPPIV activity compared to the weak or nonbiofilm formers. Interestingly, our study is the first to show that DPPIV activity even increased during biofilm formation, which might suggest that biofilm development promotes bacterial survival through the upregulation of enzyme activity. The molecular basis of the high DPPIV activity upon biofilm formation should be further elucidated at the gene and protein expression level. A recent gene expression study using DNA microarrays showed that several genes involved in growth and metabolism are downregulated, while a number of virulence determinants were highly upregulated when P. gingivalis grows as a biofilm (Lo et al., 2009). The augmented DPPIV activity in our biofilms might be related to its importance for bacterial virulence in the oral biofilm, as the proteolytic action of DPPIV contributes to the nutritional requirements of P. gingivalis and the colonization of the oral cavity through tissue disruption. Kumagai et al. (2000) have already established DPPIV as a P. gingivalis virulence factor using DPPIV-deficient mutants in a murine abscess model. Infection with the wild-type strains caused more severe abscess formation and increased mortality compared to DPPIV-deficient mutants (Kumagai et al., 2000). Also in our study, a murine dorsal subcutaneous abscess model was used to compare the pathogenic potential of the clinical isolates. Although this model does not reproduce all aspects of human periodontitis, it provides a convenient approach to evaluate bacterial virulence. Only the good biofilm formers with a high DPPIV activity induced abscesses, which may further indicate the potential role of these bacterial properties for the pathogenicity of P. gingivalis. Experimental conditions determine the absolute quantitative outcomes of in vitro assessments. In our setup, different growth media resulted in different absolute DPPIV activities, as shown in Figs 3 and 4, indicating the importance of standardization in this type of experiments. However, the relative differences in DPPIV activities between the clinical isolates were independent of the growth medium. Also biofilm formation, which was quantified by absorbance measurement after crystal violet staining, suffered from some variability due to minor differences in the experimental circumstances. To handle this variability, P. gingivalis reference strains were used to allow relative assessment of biofilm growth. Overall, the biofilm-forming capacity of the reference strains was in line with literature, that is, ATCC 33277, W50, and W83 formed good, moderate, and weak biofilms, respectively. ATCC 33277 proved to form biofilms, but showed only low DPPIV activity. In vivo, this strain was nonpathogenic, which further supports the importance of DPPIV in the full package of virulence factors for pathogenicity. The fact that ATCC 33277 forms biofilms despite its low DPPIV activity is in line with the concept that DPPIV is upregulated in biofilms, but not directly contributes to biofilm formation. However, we can hypothesize that membrane-associated DPPIV enhances biofilm growth by acting as an adhesin, which has already been described for proteases such as gingipains (Tokuda et al., 1996; Grenier et al., 2003). Many bacterial surface structures, such as fimbriae, outer membrane proteins and lipopolysaccharides, influence bacterial hydrophobicity and therefore surface attachment and biofilm formation (Dierickx et al., 2003; Grenier et al., 2003). The difference in biofilm-forming capacity of the clinical isolates could also be related to their difference in capsular type, as this significantly contributes to the bacterial surface properties (Dierickx et al., 2003; Davey, 2006; Biyikoglu et al., 2012). Our straightforward method to assess biofilm growth in vitro allows the evaluation of the biofilm-forming capacity of additional clinical isolates with different capsular types to clarify the importance of the capsular type for biofilm growth (Davey, 2006). Overall, our data obtained in clinical isolates are in accordance with literature data and strengthen the hypothesis that biofilm formation and DPPIV activity are important tools for P. gingivalis pathogenicity (Yagishita et al., 2001; Kumagai et al., 2005). As such, these bacterial factors could be considered as potential therapeutic targets for virulence inhibition. Given the increasing number of reports on resistance to current antibiotics and the lack of new drugs in the developmental pipeline, alternative therapies to improve the eradication of bacterial infections are needed (Escaich, 2008; Rasko & Sperandio, 2010). In conclusion, biofilm formation, DPPIV activity, and in vivo pathogenicity strongly correlate in our clinical P. gingivalis isolates. Moreover, our data suggest that the biofilm mode of growth may improve the virulence potential of P. gingivalis by upregulation of other virulence factors such as DPPIV. Considering the key roles of biofilm formation and bacterial proteases for oral colonization and growth, these virulence factors could present interesting targets to tackle periodontitis. Acknowledgements This project was supported by the Agency for Innovation by Science and Technology in Flanders (IWT, Belgium), the University of Antwerp (BOF-GOA, ID: 25624), and the Fund for Scientific Research (FWO-Flanders, Belgium, Project code G.0173.09N). S.C. is a Ph.D. fellow of the IWT-Flanders. We thank Pim-Bart Feijens for his support during the in vivo assays.