File - Valerie Miller E
advertisement
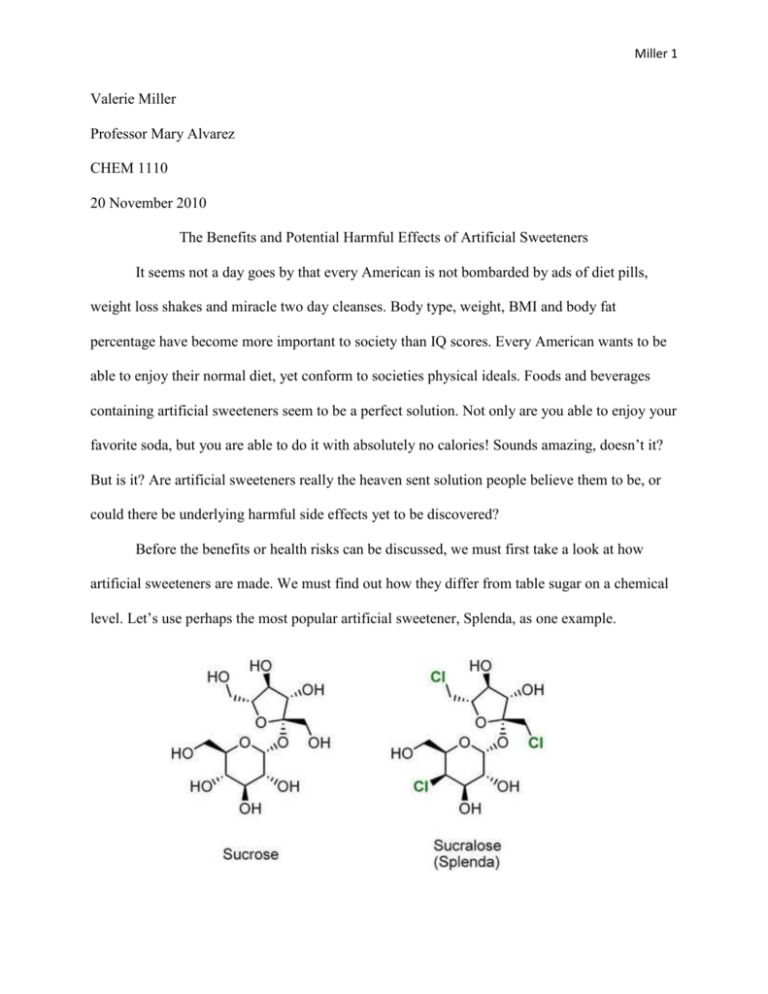
Miller 1 Valerie Miller Professor Mary Alvarez CHEM 1110 20 November 2010 The Benefits and Potential Harmful Effects of Artificial Sweeteners It seems not a day goes by that every American is not bombarded by ads of diet pills, weight loss shakes and miracle two day cleanses. Body type, weight, BMI and body fat percentage have become more important to society than IQ scores. Every American wants to be able to enjoy their normal diet, yet conform to societies physical ideals. Foods and beverages containing artificial sweeteners seem to be a perfect solution. Not only are you able to enjoy your favorite soda, but you are able to do it with absolutely no calories! Sounds amazing, doesn’t it? But is it? Are artificial sweeteners really the heaven sent solution people believe them to be, or could there be underlying harmful side effects yet to be discovered? Before the benefits or health risks can be discussed, we must first take a look at how artificial sweeteners are made. We must find out how they differ from table sugar on a chemical level. Let’s use perhaps the most popular artificial sweetener, Splenda, as one example. Miller 2 As you can see from the diagram above, Splenda is looks chemically similar to sugar, except for the addition of three atoms of chlorine. I also wanted to include a diagram of one of the most controversial artificial sweeteners, aspartame. As you can see, the chemical structure of aspartame is considerably different that sugar, in that it has the addition of both carbon and nitrogen. Sucrose has the chemical structure of ( C12 H22 O11). Splenda has the chemical structure of (C11 H19 O8 Cl3), replacing three chloride groups for three hydroxyl groups. Aspartame has the chemical structure of (C13 H18 O5 N2). (Chemistry Explained: Foundations and Applications) Artificial sweeteners are relatively easy to make and because of their molecular structures they are much sweeter than regular sucrose. Aspartame is 200 times sweeter than table sugar and sucralose is actually 600 times sweeter! The molecular structures of artificial sweeteners work by binding to a certain receptor molecule on the tongue. The receptor is coupled to a G-protein and a enzymatic reaction occurs. As a result of this ezymatic reaction, signals are sent to the brain which is interpreted as sweetness. The molecular shape is important to its level of sweetness. (Chemistry Explained: Foundations and Applications) Miller 3 For the purposes of this paper, I have decided to focus on sucralose and aspartame. Aspartame, marketed under the name Nutra-Sweet, was disccovered in 1965. Aspartame is a methyl ester of the aspartic acid/phenylalaline dipeptide . It is approximately 200 times sweeter than table sugar. It was approved for use by the FDA in 1974. However, patients with PKU should not contain aspartame because of its inclusion of phenylalaline. (Wikipedia 2009) Aspartame is one of the most highly critized artificial sweetener because of its chemistry. This is because when aspartame enters the body it is hydrolysed to three different chemicals: aspartic acid, phenylalanine, and menthol. Aspartic acid and phenylalanine are amino acids Phenylalaline is found in the brain. Aspartate is an neurotransmitter, which is high levels can actually kill other neurons and allow for the build up of free radicals. Aspartic acid and phenylalaline in extremely high does can be fatal. Menthol is highly toxic. It never appears alone, however, it is always accompanied by ethanol, which helps lower the toxicity. (Harrison 2001) Controversy over the safety of aspartame has been the topic of my debates. For now, the FDA concludes that aspartame is safe for consumption. Over 90 other countries also use aspartame and believe it to be safe. (Wikipedia ) However, there are arguments against its safety. In 2005, a study in which rats were given doses of aspartame, some developed leukemia and lymphomas. There have also been studies that have seen a correlation between the use of aspartame and an increase in brain tumors, although none of these claims have been proven. (National Cancer Institute) Perhaps the most commonly used artificial sweetener is sucralose, commonly known as Splenda. Sucralose was discovered in 1976 and approved by the FDA in 1999. It is 600 times sweeter than regular sugar. It is commonly used in diet soft drinks. Unlike aspartame, sucralose Miller 4 is very stable under high heat and over many different pH conditions, so it can be used in cooking. Sucralose is marketed as being made from sugar, however some believe that this is deceiving to consumers because they believe they are consuming a natural product. Sucralose is not natural and its chemical structure is significantly different. Sucralose contains three chloride groups instead of three hydroxyl groups. (Chemistry Daily) Sucralose is manufactured by selective chlorination of sucrose, in which three hydroxyl groups are replaced by three chloride groups. Sucralose has received scrutiny because it belongs to a group of compounds known as organochlorines. This family of compounds is known to contain many carcinogens. (Chemistry Daily) Although the FDA considers sucralose to be a safe artificial sweetener, there are some that disagree. In some studies it has been found that there is a correlation between high sucralose intake and enlarged liver and kidneys. (Hull 2002) Some also are skeptical that the chlorine added to sucralose is the same as the chlorine that is added in table salt, and that consumers could be ingesting chlorine pesticides. This claim however has been completely unfounded. (Hull 2002) Whether or not there have been proven health risks to using artificial sweeteners, there are proven side effects that appear in some people who use them. Nausea, vomiting, upset stomach and dizziness have been reported in people who ingested either sucralose or aspartame. Depression and headaches have been seen in some people who used aspartame on a regular basis. Chlorine by itself is considered a carcinogen that has been used in poisonous gases and pesticides. The side effects of using sucralose could include skin irritation, rash or hives. (MedicineNet) Miller 5 It seems as I researched the negative effects of artificial sweeteners, I was bombarded with more positive, supportive research than negative. Some of the proposed benefits to using artificial sweeteners were the decrease cause of dental decay, the ability for diabetic patients to enjoy sweets without blood sugar spikes, and the use as dietary weight loss aid. Because artificial sweeteners are chemically made, they are hundreds of times more sweet than normal table sugar. Because of this, very little is needed to achieve the desired sweetness. Because very little is needed, there are very few or possible no calories. This can help dieters to be able to eat foods that they like, in a lower calorie version. This, along with a physical exercise regimen, can help people lose the weight without losing all of their favorite foods. Because it is chemically made, it is not even broken down by the body because it does not know what to do with it. The American Dental Association has noted that artificial sweeteners do not contribute to tooth decay. Artificial sweeteners are beneficial in helping patients with diabetes. It allows diabetics to control their carbohydrate intake, and enjoy some of their favorite foods without spiking their blood sugar levels. Since artificial sweeteners are low in calories, it helps diabetics to maintain and lower their weight to help their disease. (Aspartame Information Center) In doing the research for this paper, it has been discovered that chemistry truly is all around us, even down to the sugar that is in diet soda. It is important for consumers to know that artificial sugars are just that, artificial. They are manufactured and are chemically much different than general table sugar. They may taste similar, but their molecular structures are vastly different. Whenever you are dealing with a manufactured material, caution must be taken. The body does not always know how to handle these products and negative effects can occur. We must always take control of our health and thoroughly do our research. Miller 6 Bibliography Aspartame Information Center. The Benefits. 2010. 23 November 2010 <http://www.aspartame.org/aspartame_benefits.html>. Chemistry Daily . Sucralose. 4 January 2007. 23 November 2010 <http://www.chemistrydaily.com/chemistry/Sucralose>. Chemistry Explained: Foundations and Applications. Artificial Sweeteners. 2010. 23 November 2010 <http://www.chemistryexplained.com/Ar-Bo/Artificial-Sweeteners.html>. Harrison, Karl. Artificial Sweeteners. November 2001. 23 November 2010 <http://www.3dchem.com/moremolecules.asp?ID=24&othername=artificial%20sweetener>. Hull, Janet Starr. Aspartame-Other Sweeteners. 2002. 23 November 2010 <http://www.sweetpoison.com/aspartame-sweeteners.html>. MedicineNet. Artificial Sweeteners. 1996-2010. 23 November 2010 <http://www.medicinenet.com/artificial_sweeteners/page8.htm>. National Cancer Institute. Artificial Sweeteners and Cancer. 2006. 23 November 2010 <http://www.cancer.gov/cancertopics/factsheet/Risk/artificial-sweeteners>. Wikipedia . Aspartame. 24 November 2010. 23 November 2010 <http://en.wikipedia.org/wiki/Aspartame>.












