WEEK 3 Jan. 22
advertisement

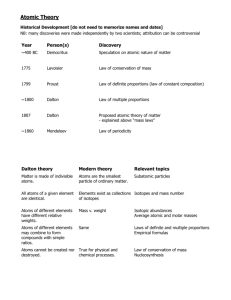
Honors Chemistry Week-At-A-Glance WEEK Goal: SC3 Atomic Structure https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Wednesday Thursday MLK, Jr. Holiday Essential Question: How is data analyzed and manipulated in chemistry? Standard: SCSh4: Students will use tools and instruments for observing, measuring, and manipulating scientific equipment and materials; SCSh5: Students will demonstrate the computation and estimation skills necessary for analyzing data and developing reasonable scientific explanations. SCSh2: Use standard safety practices. Objectives: Solve multi-step dimensional analysis problems Apply concepts of metric units, scientific notation, sig figs, & dimensional analysis in lab Essential Question: How is data analyzed and manipulated in chemistry? Standard: SCSh4: Students will use tools and instruments for observing, measuring, and manipulating scientific equipment and materials; SCSh5: Students will demonstrate the computation and estimation skills necessary for analyzing data and developing reasonable scientific explanations. SCSh2: Use standard safety practices. Objectives: Solve multi-step dimensional analysis problems Apply concepts of metric units, scientific notation, sig figs, & dimensional analysis in lab Unit One Lab Practical Unit One Cumulative Test 3 Jan. 22 - 25 Friday Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3a: Discriminate between the relative size, charge, and position of protons, neutrons and electrons in the atom. SC3c: Explain the relationship of the proton number to the element’s identity. SC3d: Explain the relationship of isotopes to the relative abundance of atoms of a particular element. Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3a: Discriminate between the relative size, charge, and position of protons, neutrons and electrons in the atom. SC3c: Explain the relationship of the proton number to the element’s identity. SC3d: Explain the relationship of isotopes to the relative abundance of atoms of a particular element. Objectives: Review the basic structure of the atom Understand the significance of each subatomic particle Use relationships between particles to make predictions about the basic structure Understand concept of isotopes Calculate atomic mass Visual/Auditory Activity: Models of the Atom, Isotopes, and Atomic Structure Numbers Formative Assessment: Atomic structure numbers exit questions Homework: Atomic Structure Numbers Practice Objectives: Review the basic structure of the atom Use relationships between particles to make predictions about the basic structure Understand concept of isotopes Calculate atomic mass Self-Assessment: Check Atomic Structure Numbers Practice Visual/Auditory Activity: Introduction to atomic mass calculations; Understanding the difference between atomic mass & mass number Independent Practice: Atomic Mass Calculations Honors Chemistry Week-At-A-Glance WEEK 4 Goal: SC3 Atomic Structure https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Wednesday Thursday Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3a: Discriminate between the relative size, charge, and position of protons, neutrons and electrons in the atom. SC3c: Explain the relationship of the proton number to the element’s identity. SC3d: Explain the relationship of isotopes to the relative abundance of atoms of a particular element. Objectives: Review the basic structure of the atom Use relationships between particles to make predictions about the basic structure Understand concept of isotopes Calculate atomic mass Formative Assessment: Basic Structure and Isotopic Notation Quiz Kinesthetic Activity: Candium Lab Visual/Auditory Activity: Wavelength, frequency, & energy calculations Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3a: Discriminate between the relative size, charge, and position of protons, neutrons and electrons in the atom. SC3c: Explain the relationship of the proton number to the element’s identity. SC3d: Explain the relationship of isotopes to the relative abundance of atoms of a particular element. Objectives: Review the basic structure of the atom Understand the significance of each subatomic particle Use relationships between particles to make predictions about the basic structure Understand concept of isotopes Calculate atomic mass Formative Assessment: Atomic Mass Quiz Self-Assessment: Go over wavelength, frequency, & energy calculations Kinesthetic Activity: Labeling the periodic table for electron configuration Visual/Auditory Activity: Electron configuration Homework: Wavelength, frequency, and energy calculations Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3a: Discriminate between the relative size, charge, and position of protons, neutrons and electrons in the atom. SC3c: Explain the relationship of the proton number to the element’s identity. SC3d: Explain the relationship of isotopes to the relative abundance of atoms of a particular element. Objectives: Review the basic structure of the atom Understand the significance of each subatomic particle Use relationships between particles to make predictions about the basic structure Understand concept of isotopes Calculate atomic mass DUE: Candium Lab Formative Assessment: Energy calculations quiz Self-Assessment: Go over Atomic Mass Quiz Visual/Auditory Activity: Noble gas notation & drawing orbital diagrams Homework: Read pgs. 377384. Answer Practice Problems 11.2 and 11.3 Jan. 28 – Feb. 1 Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3b: Use the orbital configuration of neutral atoms to explain its effect on the atom’s chemical properties. SC3f: Relate light emissions and movement of electrons to the identification of the element. Friday Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3b: Use the orbital configuration of neutral atoms to explain its effect on the atom’s chemical properties. SC3f: Relate light emissions and movement of electrons to the identification of the element. Objectives: Understand the arrangement of electrons in the atom (aufbau, Pauli, and Hund’s rules) Describe the quantum mechanical model of the atom Objectives: Illustrate the orbital configuration of electrons in atoms Associate orbital diagrams with specific locations of electrons within atoms Formative Assessment: Electron Configuration quiz Kinesthetic Activity: Flame test experiments and reports Homework: Electron configuration & orbital diagram practice Self-Assessment: Go over Energy Calculations Quiz Self-Monitoring: Success with electron configuration & orbital diagram Guided Remediation: Atomic Mass & abundance algebra Visual/Auditory Activity: Using orbital diagrams to predict exceptions to aufbau rule Homework: page 393 27, 3040 Honors Chemistry Week-At-A-Glance WEEK Goal: SC3 Atomic Structure https://sites.google.com/site/harrisonhighchemistrytarvin/ Monday Tuesday Wednesday Thursday Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Essential Question: What’s wrong with Jimmy Neutron’s model of the atom? Standard: SC3b: Use the orbital configuration of neutral atoms to explain its effect on the atom’s chemical properties. SC3f: Relate light emissions and movement of electrons to the identification of the element. Standard: SC3b: Use the orbital configuration of neutral atoms to explain its effect on the atom’s chemical properties. SC3f: Relate light emissions and movement of electrons to the identification of the element. Objectives: Understand the arrangement of electrons in the atom (aufbau, Pauli, and Hund’s rules) Describe the quantum mechanical model of the atom Objectives: Illustrate the orbital configuration of electrons in atoms Stability & exceptions to aufbau principle Unit Two Lab Practical Unit Two Cumulative Test 5 Feb. 4 - 8 Friday