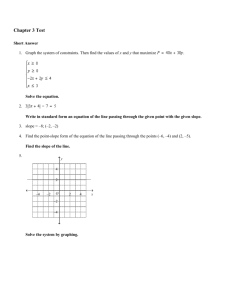
Final Review Part II Answer Section
advertisement
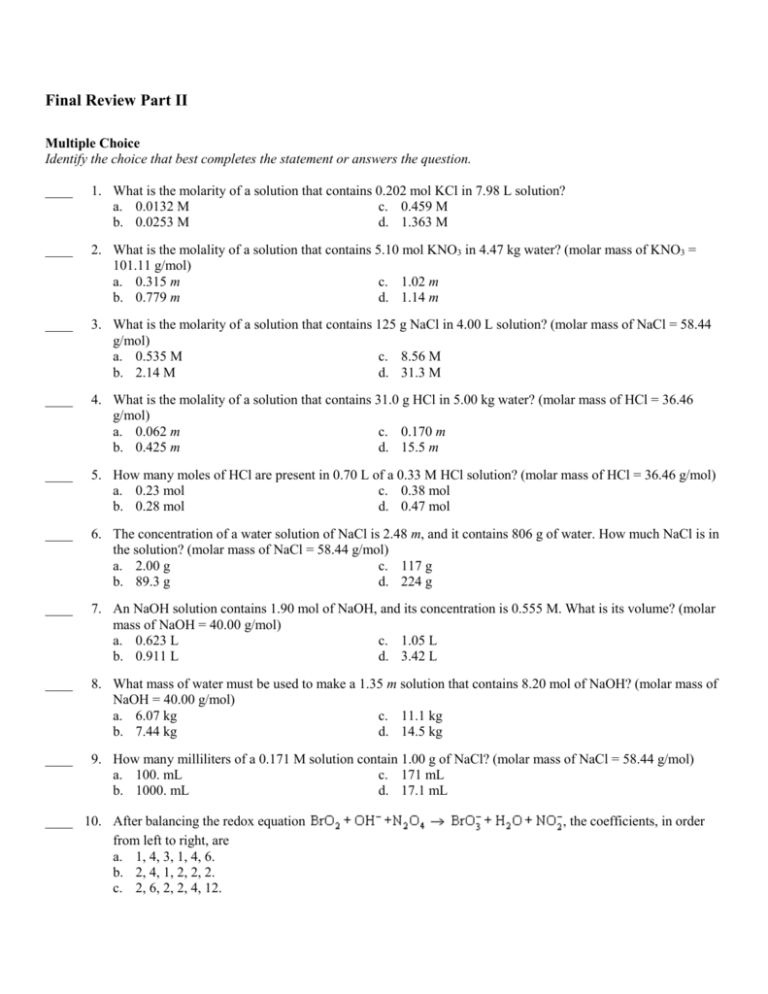
Final Review Part II Multiple Choice Identify the choice that best completes the statement or answers the question. ____ 1. What is the molarity of a solution that contains 0.202 mol KCl in 7.98 L solution? a. 0.0132 M c. 0.459 M b. 0.0253 M d. 1.363 M ____ 2. What is the molality of a solution that contains 5.10 mol KNO3 in 4.47 kg water? (molar mass of KNO3 = 101.11 g/mol) a. 0.315 m c. 1.02 m b. 0.779 m d. 1.14 m ____ 3. What is the molarity of a solution that contains 125 g NaCl in 4.00 L solution? (molar mass of NaCl = 58.44 g/mol) a. 0.535 M c. 8.56 M b. 2.14 M d. 31.3 M ____ 4. What is the molality of a solution that contains 31.0 g HCl in 5.00 kg water? (molar mass of HCl = 36.46 g/mol) a. 0.062 m c. 0.170 m b. 0.425 m d. 15.5 m ____ 5. How many moles of HCl are present in 0.70 L of a 0.33 M HCl solution? (molar mass of HCl = 36.46 g/mol) a. 0.23 mol c. 0.38 mol b. 0.28 mol d. 0.47 mol ____ 6. The concentration of a water solution of NaCl is 2.48 m, and it contains 806 g of water. How much NaCl is in the solution? (molar mass of NaCl = 58.44 g/mol) a. 2.00 g c. 117 g b. 89.3 g d. 224 g ____ 7. An NaOH solution contains 1.90 mol of NaOH, and its concentration is 0.555 M. What is its volume? (molar mass of NaOH = 40.00 g/mol) a. 0.623 L c. 1.05 L b. 0.911 L d. 3.42 L ____ 8. What mass of water must be used to make a 1.35 m solution that contains 8.20 mol of NaOH? (molar mass of NaOH = 40.00 g/mol) a. 6.07 kg c. 11.1 kg b. 7.44 kg d. 14.5 kg ____ 9. How many milliliters of a 0.171 M solution contain 1.00 g of NaCl? (molar mass of NaCl = 58.44 g/mol) a. 100. mL c. 171 mL b. 1000. mL d. 17.1 mL ____ 10. After balancing the redox equation from left to right, are a. 1, 4, 3, 1, 4, 6. b. 2, 4, 1, 2, 2, 2. c. 2, 6, 2, 2, 4, 12. , the coefficients, in order d. 1, 8, 3, 2, 4, 2. ____ 11. After balancing the redox equation HBr + NaMnO4 NaBr + MnBr2 + Br2 + H2O, the coefficients, in order from left to right, are a. 8, 2, 2, 2, 5, 8. b. 4, 1, 1, 1, 3, 2. c. 16, 2, 2, 2, 3, 4. d. 16, 2, 2, 2, 5, 8. ____ 12. After balancing the redox equation FeCl2 + KMnO4 + HCl FeCl3 + MnCl2 + H2O + KCl, the coefficients, in order from left to right, are a. 3, 1, 4, 3, 1, 2, 1. b. 4, 2, 5, 4, 2, 3, 2. c. 5, 1, 8, 5, 1, 4, 1. d. 5, 1, 4, 5, 1, 4, 1. ____ 13. After balancing the redox equation from left to right, are a. 1, 3, 5, 2, 3, 1. b. 1, 2, 4, 2, 2, 1. c. 2, 5, 10, 4, 5, 2. d. 2, 1, 2, 5, 1, 2. , the coefficients, in order ____ 14. After balancing the redox equation FeSO4 + KMnO4 + H2SO4 Fe2(SO4)3 + MnSO4 + K2SO4 + H2O, the coefficients, in order from left to right, are a. 5, 4, 8, 5, 1, 2, 4. b. 2, 5, 3, 2, 5, 1, 4. c. 10, 2, 8, 5, 2, 1, 8. d. 5, 1, 4, 3, 1, 1, 4. ____ 15. After balancing the redox equation in which elemental chlorine reacts with sodium hydroxide to produce sodium hypochlorite (NaClO), water, and sodium chloride, the coefficients of these substances are, respectively, a. 1, 1, 1, 1, 1. b. 1, 2, 1, 1, 1. c. 2, 4, 2, 1, 2. d. 1, 2, 1, 2, 1. ____ 16. After balancing the redox equation FeCl3 + Zn ZnCl2 + Fe, the coefficients, in order from left to right, are a. 2, 2, 1, 2. b. 1, 1, 1, 1. c. 4, 3, 3, 4. d. 2, 3, 3, 2. ____ 17. After balancing the redox equation left to right, are a. 4, 5, 4, 6, 6. b. 2, 3, 2, 3, 3. c. 3, 5, 3, 5, 5. d. 2, 1, 2, 1, 3. , the coefficients, in order from Use the table below to answer the following questions. Relative Strength of Oxidizing and Reducing Agents Reducing Agents Oxidizing Agents S Li Li+ W + T K K E R Ca Ca2+ A O Na Na+ K 2+ N Mg Mg E G Al Al3+ R 2+ E Zn Zn R Cr Cr3+ Fe Fe2+ Ni Ni2+ Sn Sn2+ Pb Pb2+ H2 H3O+ H2S S Cu Cu2+ I– I2 MnO42– MnO4– Fe2+ Fe3+ Hg Hg22+ Ag Ag+ NO2– NO3– S – Br Br2 T W Mn2+ MnO2 R E SO2 H2SO4 (conc.) O A Cr3+ Cr2O72– N – K Cl Cl2 G 2+ – E Mn MnO4 E R F– F2 R ____ 18. What ion oxidizes Sn to Sn2+ but does not oxidize Hg to Hg22+? a. NO3– c. b. Al3+ d. Cu2+ ____ 19. What ion is reduced by Zn but reduces Ag+ to Ag? a. Mg2+ c. Fe2+ b. Fe3+ d. ____ 20. What element displaces Cu2+ ions from solution but is displaced by Ni metal when it is in ionic form? a. Al c. Fe b. Ag d. Pb ____ 21. Copper, in each of the reactions represented by equations that are part of the lightening step of the photochromic glass photo-oxidation process, is a(n) i. Cl + Cu+ ? Cu+2 + Cl- ii. a. b. c. d. Cu+2 + Ag ? Cu+1 + Ag+ reducing agent only. oxidizing agent only. reducing agent and an oxidizing agent. neutralizing agent only. Use the table below to answer the following questions. Half-cell reaction Standard Reduction Potentials Standard Half-cell reaction electrode potential, E0 (in volts) F2 + 2e– F– MnO4– + 8H+ + 5e– Mn2+ + 4H2O 3+ – Au + 3e Au Cl2 + 2e– 2Cl– Cr2O72– + 14H+ + 6e– 2Cr3+ + 7H2O + – MnO2 + 4H + 2e Mn2+ + 2H2O – – Br2 + 2e 2Br 2+ – Hg + 2e Hg Ag+ + e– Ag Hg22+ + 2e– 2Hg 3+ – 2+ Fe + e Fe MnO4– + e– MnO42– – – I2 + 2e 2I 2+ – Cu + 2e Cu Cu2+ + e– Cu+ S + 2H2+(aq) + 2e– H2S(aq) + – 2H (aq) + 2e H2 +2.87 +1.50 +1.50 +1.36 +1.23 +1.22 +1.07 +0.85 +0.80 +0.80 +0.77 +0.56 +0.54 +0.34 +0.15 +0.14 0.00 Fe3+ + 3e– Fe 2+ – Pb + 2e Pb Sn2+ + 2e– Sn Ni2+ + 2e– Ni Co2+ + 2e– Co Cd2+ + 2e– Cd Fe2+ + 2e– Fe S2+ + 2e– S2– 3+ – Cr + 3e Cr Zn2+ + 2e– Zn 3+ – Al + 3e Al Mg2+ + 2e– Mg Na+ + e– Na Ca2+ + 2e– Ca Ba2+ + 2e– Ba K + + e– K Li+ + e– Li Standard electrode potential, E0 (in volts) –0.04 –0.13 –0.14 –0.26 –0.28 –0.40 –0.45 –0.48 –0.74 –0.76 –1.66 –2.37 –2.71 –2.87 –2.91 –2.93 –3.04 ____ 22. Calculate E0 for the spontaneous reaction when an Ag+/Ag half-cell is joined to an Hg2+/Hg half-cell. Name the neutral metal produced. a. +1.65 V; Ag c. +0.05 V; Ag b. +1.65 V; Hg d. +0.05 V; Hg ____ 23. Calculate E0 for the spontaneous reaction when a Co2+/Co half-cell is joined to a Cu2+/Cu half-cell. Name the neutral metal produced. a. +0.62 V; Cu c. +0.06 V; Cu b. +0.62 V; Co d. +0.06 V; Co ____ 24. Calculate E0 for the reaction 3Ni2+ + 2Cr 3Ni + 2Cr3+. Is the reaction spontaneous? a. –1.00 V; yes c. +0.48 V; yes b. –0.48 V; no d. +0.48 V; no ____ 25. What is the half-life of an isotope if 125 g of a 500 g sample of the isotope remain after 3.0 years? a. 1.5 years c. 3.5 years b. 2.5 years d. 4.5 years ____ 26. According to the table below, if a rock contains 25% as much uranium-235 as rocks being formed today, how old is the rock? Nuclide Half-Life (years) carbon-14 5.71 103 potassium-40 1.26 109 radium-226 1.60 103 thorium-230 7.54 104 uranium-235 7.04 108 a. 7.04 108 years b. 3.55 108 years c. 2.84 109 years d. 1.41 109 years ____ 27. Which of the following has components in a nonuniform arrangement? a. homogeneous mixture c. salt water b. solution d. heterogeneous mixture ____ 28. A heterogeneous mixture always contains a. only one substance. b. more than two substances. c. two or more substances that are visibly distinguishable. d. two or more substances that are not visibly distinguishable. ____ 29. Which of the following is a homogeneous mixture of substances in a single phase? a. a solution c. a compound b. a colloid d. a suspension ____ 30. Which is not an example of a colloid? a. paint b. smoke c. butter d. sugar water ____ 31. Which mixture contains visible particles that settle out unless the mixture is stirred? a. a colloid c. a solution b. a homogeneous mixture d. a suspension ____ 32. Which mixture contains particles that are in a dispersed phase and do not settle out? a. a colloid c. a solution b. a homogeneous mixture d. a suspension ____ 33. A metal solution is a(n) a. colloid. b. alloy. c. suspension. d. emulsion. ____ 34. A foam is a colloidal dispersion of a. two liquids. b. two solids. c. a solid and a liquid. d. a gas and a liquid. ____ 35. The colloidal particles in a colloid form the a. dispersing medium. b. dispersed phase. c. solvent. d. solute. ____ 36. Colloids a. can be separated by filtering. b. settle out when allowed to stand. c. scatter light. d. contain particles larger than 1000 nm. ____ 37. The Tyndall effect is used to distinguish between a. liquids and gases. c. electrolytes and nonelectrolytes. b. solutions and colloids. d. solvents and solutes. ____ 38. A substance whose water solution is a good conductor of electricity is a(n) a. nonelectrolyte. c. nonpolar substance. b. electrolyte. d. solute. ____ 39. An apparatus for testing conductivity is placed in a solution. The power supply is turned on and the light bulb glows brightly. This indicates that the solution a. is heterogeneous. c. contains an electrolyte. b. is supersaturated. d. is saturated. ____ 40. A substance whose water solution is a poor conductor of electricity is a(n) a. polar substance. c. electrolyte. b. nonelectrolyte. d. ionic substance. ____ 41. Substances whose water solutions conduct electricity easily a. require carbon to decompose in water. b. ionize in water. c. do not dissolve in water. d. contain neutral solute molecules. ____ 42. Which of the following does not increase the rate of dissolving a solid in water? a. raising the temperature of the water b. stirring the solution c. using larger pieces of solid d. crushing the solid ____ 43. Increasing the surface area of the solute a. increases the rate of dissolution. b. decreases the rate of dissolution. c. has no effect on the rate of dissolution. d. can increase, decrease, or have no effect on the rate of dissolution. ____ 44. Which of the following decreases the average kinetic energy of solvent molecules? a. decreasing the pressure b. not stirring the solution c. decreasing the contact area between solvent and solute d. decreasing the temperature ____ 45. Stirring increases the rate of dissolution because it a. raises the temperature. b. lowers the temperature. c. brings fresh solvent into contact with the solute. d. decreases the surface area of the solute. ____ 46. Which of the following will dissolve most rapidly? a. sugar cubes in cold water c. powdered sugar in cold water b. sugar cubes in hot water d. powdered sugar in hot water ____ 47. Which of the following will dissolve most slowly? a. large salt crystals in unstirred water c. small salt crystals in unstirred water b. large salt crystals in stirred water d. small salt crystals in stirred water ____ 48. Raising the collision rate between solute and solvent a. increases the rate of dissolution. b. decreases the rate of dissolution. c. has no effect on the rate of dissolution. d. can increase, decrease, or have no effect on the rate of dissolution. ____ 49. Raising solvent temperature causes solvent-solute collisions to become a. less frequent and more energetic. c. less frequent and less energetic. b. more frequent and more energetic. d. more frequent and less energetic. ____ 50. If a solution is not agitated while it is being made, dissolved solute tends to a. mix uniformly. c. build up in the solvent near the solute. b. build up in the solvent far from the solute. d. raise the temperature of the solvent. ____ 51. Which of the following is at equilibrium when undissolved solute is visible? a. a saturated solution c. a supersaturated solution b. an unsaturated solution d. All of the above ____ 52. If the amount of solute present in a solution at a given temperature is less than the maximum amount that can dissolve at that temperature, the solution is said to be a. saturated. c. supersaturated. b. unsaturated. d. concentrated. ____ 53. A solute crystal is dropped into a solution containing dissolved solute. It falls to the bottom of the beaker and does not dissolve after vigorous stirring. What does this indicate about the solution? a. It is probably unsaturated. c. It is probably saturated. b. It is probably supersaturated. d. It is not at equilibrium. ____ 54. In a solution at equilibrium, a. no dissolution occurs. b. the rate of dissolution is less than the rate of crystallization. c. the rate of dissolution is greater than the rate of crystallization. d. the rate of dissolution and the rate of crystallization are equal. ____ 55. The solubility of a substance at a given temperature is generally expressed as a. amount of solute. b. amount of solvent. c. amount of solute per amount of solvent. d. amount of water per 100 g of solute. ____ 56. Solubilities, expressed as grams/100. g of water, a. must be determined experimentally. b. can be easily predicted. c. vary directly with the rate at which solids dissolve. d. do not vary with temperature. ____ 57. Which of the following is likely to produce crystals if disturbed? a. an unsaturated solution c. a saturated solution b. a supersaturated solution ____ 58. The rate of dissolution is a. directly related to solubility. b. inversely related to solubility. d. an electrolytic solution c. related to the square of the solubility. d. not related to solubility. ____ 59. In the expression "like dissolves like," the word like refers to similarity in molecular a. mass. c. energy. b. size. d. polarity. ____ 60. "Like dissolves like" is a very general rule used for predicting whether a. one substance will form a solution with another. b. one substance will react with another. c. a reaction will reach equilibrium. d. a mixture will contain two or three phases. ____ 61. In a solution, the slightly charged part of a water molecule a. attracts the ions in ionic compounds. b. forms ionic bonds with ions in ionic compounds. c. attracts nonpolar molecules. d. forms ionic bonds with other water molecules. ____ 62. The solubility of ethanol, CH3CH2OH, molecules in water is enhanced by a. the pressure of the solvent. b. the size of the solute molecules. c. attraction between the ions in solution. d. hydrogen bonding between the solute and solvent molecules. ____ 63. Two immiscible substances a. exist together in one phase. b. will not separate on standing. c. dissolve freely in one another in any proportion. d. will not form a solution. ____ 64. Which of the following is a solvent for both polar and nonpolar solutes? a. water c. ethanol b. carbon tetrachloride d. benzene ____ 65. An endothermic dissolution process a. absorbs energy as heat and has positive enthalpy of solution. b. releases energy as heat and has positive enthalpy of solution. c. absorbs energy as heat and has negative enthalpy of solution. d. releases energy as heat and has negative enthalpy of solution. ____ 66. During the dissolving process, which particles interact? a. solute only c. solute and solvent b. solvent only d. None of the above ____ 67. Which of the following releases energy? a. overcoming solute-solute attraction b. forming solute-solvent attraction c. overcoming solvent-solvent attraction d. All of the above ____ 68. When the energy released by forming solvent-solute attractions is greater than the energy absorbed by overcoming solute-solute and solvent-solvent attractions, the dissolving process a. has a negative enthalpy of solution. b. has a positive enthalpy of solution. c. is endothermic. d. does not occur. ____ 69. Increasing temperature favors dissolution when a. the enthalpy of solution is negative. c. dissolution occurs rapidly. b. the enthalpy of solution is positive. d. the dissolution process is exothermic. ____ 70. The dissolution of gases in liquids is generally a. endothermic. c. rapid. b. exothermic. d. impossible. ____ 71. Enthalpy of solution is generally expressed in a. kilocalories. b. moles of solute per kilogram. c. kilojoules per mole of solute at a specified temperature. d. moles of solute in a specified amount of solvent per kilojoule. ____ 72. The formation of solid-liquid solutions a. always releases energy as heat. b. always absorbs energy as heat. c. can either absorb or release energy as heat. d. neither absorbs nor releases energy as heat. ____ 73. Pressure has the greatest effect on the solubility of a. solids in liquids. c. gases in gases. b. liquids in liquids. d. gases in liquids. ____ 74. The solubility of gases in liquids a. increases with increasing pressure. b. cannot reach equilibrium. c. decreases with increasing pressure. d. does not depend on pressure. ____ 75. Henry's law relates a. pressure to temperature. b. pressure to gas-liquid solubility. c. temperature to gas-liquid solubility. d. pressure to liquid-solid solubility. ____ 76. For a mixture of gases, the solubility of each gas in water varies a. directly with the partial pressure of the gas. b. inversely with the partial pressure of the gas. c. directly with the total pressure of the mixture. d. inversely with the total pressure of the mixture. ____ 77. Effervescence is the a. dissolution of gas in liquid. b. escape of gas from a gas-liquid solution. c. escape of liquid from a liquid-liquid solution. d. escape of solid from a solid-liquid solution. ____ 78. As temperature increases, solubility of gases in liquids a. increases. c. can increase or decrease. b. decreases. d. is not affected. ____ 79. As temperature increases, solubility of solids in liquids a. always increases. c. usually increases. b. always decreases. d. usually decreases. ____ 80. Which of the following expresses concentration? a. molality c. moles of solute per liter of solution b. molarity d. All of the above ____ 81. Which of the following is expressed in grams of solute instead of moles of solute? a. molality c. Neither (a) nor (b) b. molarity d. Both (a) and (b) ____ 82. What is the oxidation number of a monatomic ion? a. 0 c. its charge b. +1 d. its number of electrons ____ 83. What is the most common oxidation number of combined oxygen? a. –2 c. 0 b. –1 d. +1 ____ 84. What is the most common oxidation number of combined hydrogen? a. –2 c. 0 b. –1 d. +1 ____ 85. The algebraic sum of the oxidation numbers of the atoms in a compound a. is always zero. c. is always –1. b. is always +1. d. can be any whole number. ____ 86. The oxidation number of phosphorous in a. –1. b. 0. is c. +5. d. +6. ____ 87. The oxidation number for each atom in ZnCl2 is a. +1, –1. c. +2, –1. b. –2, +2. d. +2, –2. ____ 88. The oxidation number for each atom in SO3 is a. +2, –2. c. +6, –6. b. –2, +2. d. +6, –2. ____ 89. The oxidation number for each atom in HNO3 is a. –1, +2, –2. c. 0, +1, –2. b. 0, +1, –1. d. +1, +1, –2. ____ 90. The oxidation number for each atom in Al2(SO4)3 is a. +2, +4, –1. c. +3, +6, –2. b. +2, +4, –2. d. +3, +4, –1. ____ 91. The oxidation number for each atom in PbO is a. +1, –1. c. +2, –1. b. +1, –2. d. +2, –2. ____ 92. The oxidation number for each atom in CO2 is a. +3, –2. c. +4, +4. b. +4, –2. d. +3, –4. ____ 93. The oxidation number for each atom in H2SO4 is a. +1, +6, –2. b. –1, +7,–2. c. –2, +4, –2. d. +1, +6, –1. ____ 94. What is the oxidation number of a free element? a. its group number c. +1 b. its total number of valence electrons d. 0 ____ 95. In oxidation, atoms or ions a. increase their oxidation number. b. decrease their oxidation number. c. do not change their oxidation number. d. have a zero oxidation number after the reaction. ____ 96. In reduction, atoms or ions a. increase their oxidation number. b. decrease their oxidation number. c. do not change their oxidation number. d. have a zero oxidation number after the reaction. ____ 97. Which of the following are numbers assigned to atoms and ions to keep track of electrons? a. charges c. ions b. coefficients d. oxidation numbers ____ 98. A species whose oxidation number decreases in a reaction is a. oxidized. c. electrolyzed. b. reduced. d. autooxidized. ____ 99. A species whose oxidation number increases in a reaction is a. oxidized. c. electrolyzed. b. reduced. d. autooxidized. ____ 100. The conversion of a. oxidation. b. reduction. is ____ 101. The conversion of a. oxidation. b. reduction. is c. neutralization. d. disproportionation. c. neutralization. d. disproportionation. ____ 102. The loss of one or more electrons from an atom is called a. oxidation. c. electrochemistry. b. reduction. d. half-reaction. ____ 103. The gain of electrons is called a. oxidation. b. reduction. c. electrochemistry. d. half-reaction. ____ 104. Oxidation and reduction a. always occur simultaneously. b. always occur at different times. c. do not occur in the same reaction. d. always occur with oxidation first, then reduction. ____ 105. If species change their oxidation numbers, the process is a(n) a. synthesis. c. neutralization. b. decomposition. d. oxidation-reduction reaction. ____ 106. Another name for an oxidation-reduction reaction is a(n) a. double-displacement reaction. c. neutralization reaction. b. redox reaction. d. decomposition reaction. ____ 107. In a balanced redox equation, how does the total number of reactant molecules compare with the total number of product molecules? a. The two numbers are always equal. b. Reactant molecules are always more numerous. c. Product molecules are always more numerous. d. No relationship exists between the two numbers. ____ 108. In a balanced redox equation, how does the total number of reactant atoms compare with the total number of product atoms? a. The two numbers are always equal. b. Reactant atoms are always more numerous. c. Product atoms are always more numerous. d. No relationship exists between the two numbers. ____ 109. In a balanced redox equation, how does the total charge of reactants compare with the total charge of products? a. The two totals are always equal. b. Total reactant charge is always greater. c. Total product charge is always greater. d. No relationship exists between the two totals. ____ 110. How does the number of electrons lost in an oxidation compare with the number gained in the simultaneous reduction? a. The two numbers are always equal. b. The number lost is always greater than the number gained. c. The number lost is always less than the number gained. d. No relationship exists between the two numbers. ____ 111. The half-reaction method for balancing redox equations is also known as the a. ion-neutron method. c. ion-proton method. b. ion-electron method. d. ion-ion method. ____ 112. The number of steps needed for balancing redox equations by the half-reaction method is a. 5. c. 7. b. 6. d. 8. ____ 113. During redox reactions, oxidizing agents a. increase their oxidation number. b. decrease their oxidation number. c. keep the same oxidation number. d. do not participate. ____ 114. During redox reactions, reducing agents a. increase their oxidation number. b. decrease their oxidation number. c. keep the same oxidation number. d. do not participate. ____ 115. In redox reactions, a. b. c. d. the oxidizing agent is the substance oxidized. the reducing agent is the substance oxidized. both oxidizing and reducing agents are oxidized. the reducing agent is the substance reduced. ____ 116. In redox reactions, a. the oxidizing agent is the substance reduced. b. the reducing agent is the substance reduced. c. the oxidizing agent is the substance oxidized. d. both oxidizing and reducing agents are on the same side of the chemical equation. ____ 117. When Na(s) reacts with Cl2 (g) to produce NaCl (s), Na(s) is the a. oxidizing agent. c. neutralizing agent. b. reducing agent. d. ionizing agent. ____ 118. A strong reducing agent easily gives up a. electrons. b. protons. c. electrons and protons. d. neutrons. ____ 119. Which is the most electronegative element? a. oxygen b. hydrogen c. zinc d. fluorine ____ 120. In which process does a substance act as both an oxidizing agent and a reducing agent and oxidizes itself? a. electrolysis c. autoreduction b. disproportionation d. double displacement ____ 121. What is the formula of the peroxide ion? a. O2– b. O– c. d. ____ 122. Which of the following describes the bond in a peroxide ion? a. a double bond c. somewhat unstable b. highly stable d. a triple bond ____ 123. When hydrogen peroxide decomposes, oxygen is a. reduced only. c. both oxidized and reduced. b. oxidized only. d. electrolyzed. ____ 124. When a glowing splint bursts into flames during the decomposition of H2O2, it indicates that which gas is produced? a. hydrogen c. helium b. oxygen d. nitrogen ____ 125. In which system does a spontaneous redox reaction produce electrical energy? a. electrochemical cell c. electroplating cell b. electrolytic cell d. half-cell ____ 126. If the reactants in a spontaneous energy-releasing redox reaction are in direct contact, the energy is released in the form of a. light. c. heat. b. electrical energy. d. mechanical energy. ____ 127. If the reactants in a spontaneous energy-releasing redox reaction are connected externally by a wire conductor, the energy is released in the form of a. light. c. heat. b. electricity. d. movement. ____ 128. Where does oxidation take place in an electrochemical cell? a. the anode c. the anode or the cathode b. the cathode d. the half-cell ____ 129. Where does reduction take place in an electrochemical cell? a. the anode c. the anode or the cathode b. the cathode d. the half-cell ____ 130. In an electrochemical cell, a. positive ions move toward the anode. b. positive ions move toward the cathode. c. positive ions do not move. d. positive ions become negative ions. ____ 131. The oxidation number in an anode reaction a. decreases. b. increases. c. does not change. d. None of the above ____ 132. In an electrochemical cell, the anode is the a. neutral electrode. b. electrode at which matter can gain or lose electrons. c. electrode at which matter gains electrons. d. electrode at which matter loses electrons. ____ 133. An electrochemical cell that generates electrical energy is a(n) a. galvanic cell. c. electroplating cell. b. electrolytic cell. d. None of the above ____ 134. The movement of electrons or other charged particles is described as a(n) a. electric potential. c. voltage. b. electric current. d. electrode reaction. ____ 135. In any electrochemical cell, the cathode is always the a. porous electrode. b. porous barrier. c. electrode at which matter gains electrons. d. electrode at which matter loses electrons. ____ 136. In any electrochemical cell, the anode is always the a. porous electrode. b. porous barrier. c. electrode at which matter gains electrons. d. electrode at which matter loses electrons. ____ 137. What type of cell generates electrical energy? a. electrolytic cells c. galvanic cells b. electroplating cells d. oxidation cells ____ 138. In a zinc-carbon dry cell, a. the zinc electrode is the cathode, and the carbon electrode is the anode. b. the zinc electrode is the anode, and the carbon electrode is the cathode. c. both electrodes are auto-oxidizing and serve as both cathodes and anodes. d. neither electrode can be considered a cathode or an anode. ____ 139. Which of the following is not an example of an electrochemical cell? a. alkaline cell c. electrolytic cell b. dry cell d. voltaic cell ____ 140. If the reactants of a voltaic cell are in contact, a. most of the energy produced is an electric current. b. most of the energy produced is heat. c. oxidation and reduction do not occur. d. hydrogen gas is released. ____ 141. For electricity to flow in a voltaic cell, the two half-cells must be a. connected by a wire and a porous barrier. b. completely isolated from one another. c. in the same solution. d. connected to a dry cell. ____ 142. Batteries are examples of which of the following? a. electrolytic cells c. equilibrium cells b. galvanic cells d. None of the above ____ 143. Corrosion is the disintegration of metals through a. combustion. c. oxidation. b. reduction. d. exposure. ____ 144. A corrosion cell is actually a type of a. salt bridge. b. electrode. c. galvanic cell. d. electrolytic cell. ____ 145. Which conditions make corrosion worse? a. airborne salt from the oceans b. salt spread on icy roads c. air pollutants d. All of the above ____ 146. To protect steel from corrosion, it is better to coat steel with another metal that a. does not corrode. c. does corrode. b. is expensive. d. is more durable. ____ 147. The deterioration of metals is called a. combustion. b. decomposition. c. corrosion. d. electronegativity. ____ 148. What is not needed for corrosion of metals? a. oxygen b. ions c. water d. sunlight ____ 149. The voltage of a voltaic cell is determined by the E0 value(s) of the a. half-reaction at the anode. c. half-reaction at the cathode. b. half-reactions at the cathode and anode. d. standard hydrogen electrode. ____ 150. Electrons in a voltaic cell normally flow a. from cathode to anode. b. through a porous barrier. c. in both directions through the external circuit. d. from anode to cathode. ____ 151. A cell producing an electrical potential, or voltage, means that the cell is a. galvanic. c. at equilibrium. b. electrolytic. d. unstable. ____ 152. What can electrode potentials predict about a half-reaction? a. the direction the reaction takes c. the products of a reaction b. the voltage of the reaction d. the reactants of the reaction ____ 153. A more positive value for electrode potential means the electrode is more likely to be a(n) a. anode. c. electrolytic cell. b. cathode. d. electrochemical cell. ____ 154. Based on E0 values, which metal is the most easily oxidized? a. Hg c. Pb b. Zn d. Au ____ 155. Based on E0 values, which metal is the most easily reduced? a. Hg c. Pb b. Zn d. Au ____ 156. In which cell does a current drive a nonspontaneous redox reaction? a. electrolytic cell c. electrochemical cell b. dry cell d. voltaic cell ____ 157. In an electrolytic cell, reduction occurs a. at the cathode. b. at the anode. c. at either the cathode or the anode. d. between the cathode and anode. ____ 158. In an electrolytic cell, the anode a. can be either positively or negatively charged. b. is not charged. c. is positively charged. d. is negatively charged. ____ 159. In an electrolytic cell, the cathode a. can be either positively or negatively charged. b. is not charged. c. is positively charged. d. is negatively charged. ____ 160. In an electrolytic cell, negative ions move toward the a. negative electrode, and positive ions move toward the negative electrode. b. negative electrode, and positive ions move toward the positive electrode. c. positive electrode, and positive ions move toward the positive electrode. d. positive electrode, and positive ions move toward the negative electrode. ____ 161. Which is the source of energy for an electrolytic cell? a. the reaction occurring in the electrolytic cell b. an external direct-current source, such as a battery c. ion migration in the electrolyte d. electron migration in the electrolyte ____ 162. What type of cell consumes electrical energy? a. electrolytic cells c. galvanic cells b. fuel cells d. oxidation cells ____ 163. What is needed to create chemical changes in an electrolytic cell? a. gravity c. electricity b. high temperatures d. a fuel cell ____ 164. Aluminum is obtained first from a. recycling soda cans. b. pure sodium chloride. c. bauxite. d. the electrolysis of water. ____ 165. What type of process is the electrolysis reaction? a. electrode reaction c. spontaneous b. dry cell reaction d. nonspontaneous ____ 166. Electroplating is coating a material with a. a plastic covering. b. bauxite. c. another metal. d. reducing agents. ____ 167. Which process deposits metal onto a surface? a. electrolysis b. electroplating c. autooxidation d. oxidation ____ 168. Electroplating is an application of which reaction? a. electrolytic reaction c. auto-oxidation reaction b. electrochemical reaction d. voltaic reaction ____ 169. An electroplating cell contains a solution of a. a salt of the plating metal. b. a salt of the metal of the object that is to be plated. c. a substance that does not carry an electric current. d. H2SO4. ____ 170. In an electroplating cell, the object to be plated is the a. external circuit. c. anode. b. electrolyte. d. cathode. ____ 171. In an electroplating cell, the metal used to plate the object is the a. external circuit. c. anode. b. electrolyte. d. cathode. ____ 172. In an electroplating cell, a solution of the salt of the plating metal is the a. external circuit. c. anode. b. electrolyte. d. cathode. ____ 173. In a cell used to electroplate silver onto an object, Ag+ is a. oxidized at the anode. c. oxidized at the cathode. b. reduced at the anode. d. reduced at the cathode. ____ 174. In a cell used to electroplate silver onto an object, Ag is a. oxidized at the anode. c. oxidized at the cathode. b. reduced at the anode. d. plated out at the anode. ____ 175. The oxidation-reduction reactions in a rechargeable cell are the same as in a. a voltaic cell only. c. a voltaic cell and an electrolytic cell. b. an electrolytic cell only. d. a half-cell. ____ 176. When a rechargeable cell produces electrical energy, it acts as a(n) a. fuel cell. c. voltaic cell. b. electrolytic cell. d. half-cell. ____ 177. A rechargeable cell produces energy when a. it is charging. b. it is discharging. c. its external circuit is not closed. d. the porous barrier is in place. ____ 178. When electrical energy is provided to a rechargeable cell from an external source, the cell acts as a(n) a. electrochemical cell. c. voltaic cell. b. electrolytic cell. d. half-cell. ____ 179. Electrical energy is provided to a rechargeable cell from an outside source when a. it is charging. c. its external circuit is not closed. b. it is discharging. d. the porous barrier is in place. ____ 180. What is the voltage of the standard automobile battery? a. 1.5 V c. 12 V b. 6 V d. 50 V ____ 181. When an automobile battery is charging, a. energy as heat is converted to energy of motion. b. energy of motion is converted to energy as heat. c. chemical energy is converted to electrical energy. d. electrical energy is converted to chemical energy. ____ 182. When an automobile battery is starting a car, a. energy as heat is converted to energy of motion. b. energy of motion is converted to energy as heat. c. chemical energy is converted to electrical energy. d. electrical energy is converted to chemical energy. ____ 183. Which substances react in the standard automobile battery? a. lead(IV) oxide, lead, and sulfuric acid b. copper(II) oxide, copper, and sulfuric acid c. zinc oxide, zinc, and sulfuric acid d. iron(III) oxide, iron, and sulfuric acid ____ 184. In nuclear chemistry, an atom is referred to as a(n) a. nuclide. c. nucleus. b. nucleon. d. alpha particle. ____ 185. A nuclide is identified by a. the number of protons in its nucleus. b. the number of neutrons in its nucleus. c. the number of protons and neutrons in its nucleus. d. None of the above ____ 186. What does the 101 in represent? a. the mass number b. the atomic number c. the nuclide number d. the number of neutrons ____ 187. Mass defect is the difference between the mass of a. a nucleus and its atom. b. a neutron and a proton. c. an atom and the sum of the masses of its nucleons. d. an atom and the sum of the masses of its constituent particles. ____ 188. The nuclear binding energy is released when a nucleus a. is bombarded. c. is formed from its constituent particles. b. divides. d. decays. ____ 189. What elements have relatively small nuclear binding energies per nuclear particle? a. light elements only c. heavy elements only b. elements of intermediate mass d. both light and heavy elements ____ 190. Compared with the sum of the masses of the separate particles that compose the nucleus, its mass a. is always less. c. is always the same. b. is always more. d. may be either less, more, or the same. ____ 191. Between protons in a nucleus, a. attraction due to nuclear force is greater than repulsion due to electrostatic force. b. repulsion due to electrostatic force is greater than attraction due to nuclear force. c. nuclear and electrostatic forces are balanced. d. electrostatic forces are negligible. ____ 192. Reactions that affect the nucleus of an atom are called a. fusions. c. radioactive decays. b. fissions. d. nuclear reactions. ____ 193. In a nuclear reaction, unstable nuclei change their number of protons and neutrons, a. give off large amounts of energy, and increase their stability. b. give off small amounts of energy, and increase their stability. c. give off large amounts of energy, and decrease their stability. d. give off small amounts of energy, and decrease their stability. ____ 194. The spontaneous disintegration of a nucleus into a slightly lighter and more stable nucleus, accompanied by emission of particles, electromagnetic radiation, or both, is a. nuclear fusion. c. radioactive decay. b. nuclear radiation. d. nuclear fission. ____ 195. During radioactive decay, the nucleus disintegrates into a. a lighter and more stable nucleus. c. a lighter and less stable nucleus. b. a heavier and more stable nucleus. d. a heavier and less stable nucleus. ____ 196. Which of the following processes always decreases the number of protons by an even number? a. fusion c. alpha decay b. beta decay d. fission ____ 197. Which of the following forms of radiation has the greatest penetrating power? a. alpha particles c. gamma rays b. beta particles d. positrons ____ 198. Which of the following radioactive decay processes does not reduce the atomic number of a nuclide? a. alpha decay c. positron decay b. beta decay d. electron capture ____ 199. Which of the following particles has the same mass as an electron but a positive charge and is sometimes emitted from the nucleus during radioactive decay? a. beta particle c. positron b. alpha particle d. gamma ray ____ 200. Alpha particles are a. electrons. b. helium nuclei. c. electromagnetic waves. d. neutrons. ____ 201. Beta particles are a. electrons. b. helium nuclei. c. electromagnetic waves. d. neutrons. ____ 202. Gamma rays are a. electrons. b. helium nuclei. c. electromagnetic waves. d. neutrons. ____ 203. Which of the following is the symbol for an alpha particle? a. c. b. d. ____ 204. Which of the following is the nuclear symbol for a beta particle? a. c. b. d. ____ 205. The half-life of an isotope is the time required for half the nuclei in a sample to a. undergo radioactive decay. c. undergo nuclear fusion. b. undergo nuclear fission. d. react chemically. ____ 206. How many half-lives are required for three-fourths of the nuclei of one isotope in a sample to decay? a. c. 2 b. d. 3 ____ 207. Which statement is true about half-lives? a. Different atoms of the same nuclide have different half-lives. b. Each radioactive isotope has its own half-life. c. All radioactive nuclides of an element have the same half-life. d. All radioactive nuclides have the same half-life. ____ 208. Which series consists of radioactive nuclides produced by successive radioactive decay until a stable nuclide is reached? a. parent series c. nuclide series b. half-life series d. decay series ____ 209. Which of the following is the heaviest nuclide of a decay series? a. the parent nuclide c. the last radioactive nuclide b. the daughter nuclide d. the last decaying nuclide ____ 210. What nuclides are produced when a nuclide decays? a. son nuclides c. radioactive nuclides b. daughter nuclides d. decaying nuclides ____ 211. A decay series ends with a. a stable nuclide. b. a parent nuclide. c. a fission reaction. d. a transuranium element. ____ 212. Artificial radioactive nuclides are a. found naturally in space. b. found naturally on Earth. c. not found naturally on Earth. d. nonexistent. ____ 213. Artificial radioactive nuclides are produced by a. bombarding stable nuclei with particles. b. using an accelerator to overcome the nuclear force. c. beta emission. d. fission of stable nuclides. ____ 214. How are elements artificially transmuted? a. Stable nuclei are bombarded with charged particles. b. Stable nuclei are bombarded with uncharged particles. c. Stable nuclei are bombarded with charged and uncharged particles. d. Unstable nuclei are bombarded with charged and uncharged particles. ____ 215. In an artificial transmutation, what is required to bombard nuclei with positively charged alpha particles, protons, and other ions? a. great quantities of energy c. a particle accelerator b. small quantities of energy d. Both (a) and (c) ____ 216. Some artificial radioactive isotopes can be prepared by bombarding stable nuclei with a. alpha particles. c. protons. b. beta particles. d. All of the above ____ 217. Which of the following travels fastest? a. alpha particles b. beta particles c. gamma rays d. All travel at the same speed. ____ 218. Which of the following generally have the lowest penetrating ability? a. alpha particles c. gamma rays b. beta particles d. All have the same penetrating ability. ____ 219. Which of the following has the greatest penetrating ability? a. alpha particles c. gamma rays b. beta particles d. All have the same penetrating ability. ____ 220. What unit measures radiation? a. roentgen b. rem c. megaelectron-volt d. cm3 ____ 221. A roentgen equals the amount of radiation that produces a. 2 1018 ion pairs in 1 cm3 of dry air. b. 2 1019 ion pairs in 1 cm3 of dry air. c. 2 d. 2 108 ion pairs in 1 cm3 of dry air. 109 ion pairs in 1 cm3 of dry air. ____ 222. What unit measures radiation damage to human tissue? a. roentgen c. rad b. rem d. half-life ____ 223. One rem is the quantity of ionizing radiation that does as much damage to human tissue as is done by a. 1 roentgen of high-voltage X rays. b. 100 roentgens of high-voltage X rays. c. 1 roentgen of low-voltage X rays. d. the radioactive decay of 1 kg of uranium-235. ____ 224. How are the definitions of rem and roentgen related? a. The definition of roentgen depends on the rem. b. The definition of rem depends on the roentgen. c. Both are based on damage to human tissue. d. They are not related. ____ 225. Which of the following does not detect radiation? a. film badges c. scintillation counters b. Geiger-Müller counters d. radioactive tracers ____ 226. Radioactive nuclides cause molecules in air to a. ionize. c. condense. b. fluoresce. d. radiate. ____ 227. What are often used to monitor the approximate radiation exposure of people working with radioactive materials? a. film badges c. scintillation counters b. X-ray films (radiographs) d. radioactive tracers ____ 228. Which of the following instruments detect radiation by counting electric pulses carried by gas atoms ionized by radiation? a. film badges c. scintillation counters b. Geiger-Müller counters d. radioactive tracers ____ 229. Which of the following instruments detect radiation by converting light produced in a radioactive process to an electric signal? a. film badges c. scintillation counters b. Geiger-Müller counters d. radioactive tracers ____ 230. How do radioactive nuclides affect photographic film wrapped in lightproof paper? a. They have no effect on the film. c. They melt the film. b. They disintegrate the film. d. They expose the film. ____ 231. All radioactive nuclides undergo a. chemical decomposition. b. fusion. c. radioactive decay. d. fission. ____ 232. To use radioactive dating for a substance, you must know the substance's a. melting point. c. rate of weathering or erosion. b. half-life. d. enthalpy of reaction. ____ 233. Which radioactive nuclide is used to treat cancer? a. cobalt-60 c. uranium-238 b. plutonium-239 d. radon-222 ____ 234. The isotope is not used to a. kill bacteria. b. kill cancer cells. c. kill insects that infest food. d. date rocks. ____ 235. Radioactive tracers are used to a. measure the energy of nuclear reactions. b. calculate the half-life of a nuclide. c. estimate the age of a material. d. follow the movement of substances in a system. ____ 236. Radioactive tracers in fertilizers can be used to measure a. how well the fertilizer is absorbed by plants. b. contaminants in the fertilizer. c. the chemical composition of the fertilizer. d. how plants respond to radioactivity. ____ 237. Which of the following processes produces nuclei of lower mass than the reactants? a. fission c. Both (a) and (b) b. fusion d. Neither (a) nor (b) ____ 238. Which statement does not describe fission? a. A neutron starts the process. b. Several neutrons are emitted. c. Stable, lightweight nuclei start the process. d. A very heavy nucleus splits into several medium-weight nuclei. ____ 239. If the particle that starts a nuclear reaction is also one of the products, the process is a a. chain reaction. c. nuclear fusion. b. neutron emission. d. neutron bombardment. ____ 240. Which are not products of the fission of uranium? a. neutrons c. energy b. medium-weight nuclei d. alpha particles ____ 241. Which statement about nuclear reactions is not true? a. Nuclear power plants use fission of uranium. b. In fission, the total mass of the reactants equals the total mass of the products. c. In fission, nuclei are split, and in fusion, nuclei are combined. d. Heat and light in the sun are produced by hydrogen fusion reactions. ____ 242. Which of the following is a fission reaction? a. hydrogen-2 and hydrogen-3 combining to form a helium-4 atom and a neutron b. carbon-12 and hydrogen-1 combining to form a nitrogen-13 atom c. uranium-235 absorbing a neutron and breaking into barium-141, krypton-92, and three neutrons d. a glucose molecule being metabolized with oxygen to form carbon dioxide and water ____ 243. Which of the following is a fusion reaction? a. uranium-235 absorbing a neutron and splitting into xenon-140, strontium-94, and two neutrons b. hydrochloric acid combining with sodium hydroxide to form NaCl and water c. carbon-14 decaying into nitrogen-14 and a beta particle d. curium-246 combining with carbon-12 to form nobelium-254 and four neutrons ____ 244. What device uses controlled nuclear fission to produce new radioactive substances and energy? a. synchrotron c. nuclear bomb b. nuclear reactor d. linear accelerator ____ 245. In nuclear reactors, the role of control rods is to a. accelerate neutrons. c. slow down neutrons. b. absorb neutrons. d. absorb energy. ____ 246. In reactors, the role of the moderator is to a. accelerate neutrons. b. absorb neutrons. c. slow down neutrons. d. absorb energy. ____ 247. What is the function of shielding in a nuclear reactor? a. to cool the reactor c. to absorb free neutrons b. to contain radiation d. to slow neutrons ____ 248. The energy as heat produced by a reactor is used to a. boil water for steam turbines. c. produce graphite. b. melt metal. d. produce coal. ____ 249. At present, fusion reactions a. cannot be used to produce energy in reactors. b. produce the energy in some nuclear power plants. c. produce the energy in most nuclear power plants. d. produce the energy in all recent nuclear power plants. ____ 250. Scientists are investigating the possibility of containing fusion reactions within a. steel containers. c. concrete casks. b. lead containers. d. magnetic fields. Final Review Part II Answer Section MULTIPLE CHOICE 1. ANS: B Solution: PTS: 1 STA: 5.3.B.1 2. ANS: D Solution: DIF: III REF: 3 OBJ: 1 PTS: 1 STA: 5.3.B.1 3. ANS: A Solution: DIF: III REF: 3 OBJ: 1 PTS: 1 STA: 5.3.B.1 4. ANS: C Solution: DIF: III REF: 3 OBJ: 1 PTS: 1 STA: 5.3.B.1 5. ANS: A Solution: DIF: III REF: 3 OBJ: 1 PTS: 1 STA: 5.3.B.1 6. ANS: C Solution: DIF: III REF: 3 OBJ: 2 PTS: 1 STA: 5.3.B.1 7. ANS: D Solution: DIF: III REF: 3 OBJ: 2 PTS: 1 STA: 5.3.B.1 8. ANS: A Solution: DIF: III REF: 3 OBJ: 3 PTS: 1 STA: 5.3.B.1 9. ANS: A Solution: DIF: III REF: 3 OBJ: 3 DIF: III REF: 3 OBJ: 3 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 2 DIF: III REF: 3 DIF: III REF: 3 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. PTS: STA: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: 1 5.3.B.1 B 2 D 2 C 2 A 2 C 2 B 2 D 2 B 2 D 1 C 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 5.3.C.1 1 PTS: 1 OBJ: 1 20. ANS: D OBJ: 1 21. ANS: C OBJ: 1 22. ANS: D Solution: PTS: 1 DIF: III REF: 3 PTS: 1 DIF: III REF: 3 PTS: 1 DIF: III STA: 5.3.B.1 | 5.3.C.1 23. ANS: A Solution: REF: 2 OBJ: 4 PTS: 1 DIF: III STA: 5.3.B.1 | 5.3.C.1 24. ANS: C Answer: REF: 2 OBJ: 4 PTS: 1 DIF: III STA: 5.3.B.1 | 5.3.C.1 25. ANS: A Solution: REF: 2 OBJ: 4 PTS: 1 STA: 5.6.A.6 26. ANS: D Solution: REF: 2 OBJ: 3 DIF: III 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. PTS: STA: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: 1 5.6.A.6 D 1 C 1 A 1 D 3 D 3 A 3 B 3 D 3 B 3 C 3 B 3 B 4 C 4 B 4 B 4 C 1 A 1 D 1 C 1 D DIF: III REF: 2 OBJ: 4 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 OBJ: 47. ANS: OBJ: 48. ANS: OBJ: 49. ANS: OBJ: 50. ANS: OBJ: 51. ANS: OBJ: 52. ANS: OBJ: 53. ANS: OBJ: 54. ANS: OBJ: 55. ANS: OBJ: 56. ANS: OBJ: 57. ANS: OBJ: 58. ANS: OBJ: 59. ANS: OBJ: 60. ANS: OBJ: 61. ANS: OBJ: 62. ANS: OBJ: 63. ANS: OBJ: 64. ANS: OBJ: 65. ANS: OBJ: 66. ANS: OBJ: 67. ANS: OBJ: 68. ANS: OBJ: 69. ANS: OBJ: 70. ANS: OBJ: 1 A 1 A 1 B 1 C 1 A 2 B 2 C 2 D 2 C 2 A 2 B 2 D 2 D 3 A 3 A 3 D 3 D 3 C 3 A 4 C 4 B 4 A 4 B 4 B 4 STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 71. ANS: OBJ: 72. ANS: OBJ: 73. ANS: OBJ: 74. ANS: OBJ: 75. ANS: OBJ: 76. ANS: OBJ: 77. ANS: OBJ: 78. ANS: OBJ: 79. ANS: OBJ: 80. ANS: OBJ: 81. ANS: OBJ: 82. ANS: OBJ: 83. ANS: OBJ: 84. ANS: OBJ: 85. ANS: OBJ: 86. ANS: OBJ: 87. ANS: OBJ: 88. ANS: OBJ: 89. ANS: OBJ: 90. ANS: OBJ: 91. ANS: OBJ: 92. ANS: OBJ: 93. ANS: OBJ: 94. ANS: OBJ: C 4 C 4 D 5 A 5 B 5 A 5 B 5 B 5 C 5 D 1 C 1 C 1 A 1 D 1 A 1 C 1 C 1 D 1 D 1 C 1 D 1 B 1 A 1 D 1 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.6.A.7 1 5.3.B.1 1 5.3.B.1 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 95. ANS: OBJ: 96. ANS: OBJ: 97. ANS: OBJ: 98. ANS: OBJ: 99. ANS: OBJ: 100. ANS: OBJ: 101. ANS: OBJ: 102. ANS: OBJ: 103. ANS: OBJ: 104. ANS: OBJ: 105. ANS: OBJ: 106. ANS: OBJ: 107. ANS: OBJ: 108. ANS: OBJ: 109. ANS: OBJ: 110. ANS: OBJ: 111. ANS: OBJ: 112. ANS: OBJ: 113. ANS: OBJ: 114. ANS: OBJ: 115. ANS: OBJ: 116. ANS: OBJ: 117. ANS: OBJ: 118. ANS: OBJ: 119. ANS: A 2 B 2 D 2 B 2 A 2 A 2 A 2 A 2 B 2 A 3 D 3 B 3 D 1 A 1 A 1 A 1 B 2 C 2 B 1 A 1 B 1 A 1 B 1 A 1 D PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.3 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.3.C.1 1 5.3.C.1 1 OBJ: 120. ANS: OBJ: 121. ANS: OBJ: 122. ANS: OBJ: 123. ANS: OBJ: 124. ANS: OBJ: 125. ANS: OBJ: 126. ANS: OBJ: 127. ANS: OBJ: 128. ANS: OBJ: 129. ANS: OBJ: 130. ANS: OBJ: 131. ANS: OBJ: 132. ANS: OBJ: 133. ANS: OBJ: 134. ANS: OBJ: 135. ANS: OBJ: 136. ANS: OBJ: 137. ANS: OBJ: 138. ANS: OBJ: 139. ANS: OBJ: 140. ANS: OBJ: 141. ANS: OBJ: 142. ANS: OBJ: 143. ANS: OBJ: 1 B 2 D 2 C 2 C 2 A 2 A 1 C 1 B 1 A 1 B 1 B 1 B 1 D 1 A 1 B 1 C 1 D 1 C 1 B 1 D 1 B 1 A 1 B 1 C 2 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 144. ANS: OBJ: 145. ANS: OBJ: 146. ANS: OBJ: 147. ANS: OBJ: 148. ANS: OBJ: 149. ANS: OBJ: 150. ANS: OBJ: 151. ANS: OBJ: 152. ANS: OBJ: 153. ANS: OBJ: 154. ANS: OBJ: 155. ANS: OBJ: 156. ANS: OBJ: 157. ANS: OBJ: 158. ANS: OBJ: 159. ANS: OBJ: 160. ANS: OBJ: 161. ANS: OBJ: 162. ANS: OBJ: 163. ANS: OBJ: 164. ANS: OBJ: 165. ANS: OBJ: 166. ANS: OBJ: 167. ANS: OBJ: C 2 D 2 C 2 C 2 D 2 B 3 D 3 A 3 A 3 B 3 B 3 D 3 A 1 A 1 C 1 D 1 D 1 B 1 A 1 C 1 C 2 D 2 C 3 B 3 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.6.A.6 1 5.6.A.6 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 168. ANS: OBJ: 169. ANS: OBJ: 170. ANS: OBJ: 171. ANS: OBJ: 172. ANS: OBJ: 173. ANS: OBJ: 174. ANS: OBJ: 175. ANS: OBJ: 176. ANS: OBJ: 177. ANS: OBJ: 178. ANS: OBJ: 179. ANS: OBJ: 180. ANS: OBJ: 181. ANS: OBJ: 182. ANS: OBJ: 183. ANS: OBJ: 184. ANS: OBJ: 185. ANS: OBJ: 186. ANS: OBJ: 187. ANS: OBJ: 188. ANS: OBJ: 189. ANS: OBJ: 190. ANS: OBJ: 191. ANS: OBJ: 192. ANS: A 3 A 3 D 3 C 3 B 3 D 3 A 3 C 4 C 4 B 4 B 4 A 4 C 4 D 4 C 4 A 4 A 1 C 1 B 1 D 2 C 2 D 2 A 2 A 3 D PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.4.B.1 1 5.6.A.5 1 5.6.A.5 1 5.6.A.5 1 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 3 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 DIF: I REF: 1 PTS: 1 STA: 5.6.A.6 PTS: 1 DIF: I REF: 1 DIF: I REF: 1 OBJ: 4 193. ANS: A OBJ: 4 194. ANS: C OBJ: 1 195. ANS: A OBJ: 1 196. ANS: C OBJ: 2 197. ANS: C OBJ: 2 198. ANS: B OBJ: 2 199. ANS: C OBJ: 2 200. ANS: B OBJ: 2 201. ANS: A OBJ: 2 202. ANS: C OBJ: 2 203. ANS: D OBJ: 2 204. ANS: C OBJ: 2 205. ANS: A OBJ: 3 206. ANS: C Solution: 207. 208. 209. 210. 211. 212. PTS: STA: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: ANS: OBJ: 1 5.6.A.6 B 3 D 4 A 4 B 4 A 4 C 5 STA: PTS: STA: PTS: 5.3.C.1 | 5.6.A.6 1 DIF: I 5.3.C.1 | 5.6.A.6 1 DIF: I REF: 1 REF: 2 PTS: 1 DIF: I REF: 2 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 DIF: I REF: 2 REF: 2 OBJ: 3 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 1 5.6.A.6 DIF: I PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.2.B.3 | 5.4.B.1 I REF: 2 I REF: 2 I REF: 2 I REF: 2 I REF: 2 I REF: 2 213. ANS: OBJ: 214. ANS: OBJ: 215. ANS: OBJ: 216. ANS: OBJ: 217. ANS: OBJ: 218. ANS: OBJ: 219. ANS: OBJ: 220. ANS: OBJ: 221. ANS: OBJ: 222. ANS: OBJ: 223. ANS: OBJ: 224. ANS: OBJ: 225. ANS: OBJ: 226. ANS: OBJ: 227. ANS: OBJ: 228. ANS: OBJ: 229. ANS: OBJ: 230. ANS: OBJ: 231. ANS: OBJ: 232. ANS: OBJ: 233. ANS: OBJ: 234. ANS: OBJ: 235. ANS: OBJ: 236. ANS: OBJ: 237. ANS: A 5 C 5 D 5 D 5 C 1 A 1 C 1 A 1 D 2 B 2 A 2 B 2 D 2 A 2 A 3 B 3 C 3 D 3 C 4 B 4 A 4 D 4 D 4 A 4 A PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: I REF: 2 I REF: 2 I REF: 2 I REF: 2 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: 1 DIF: I REF: 3 PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 3 I REF: 4 OBJ: 238. ANS: OBJ: 239. ANS: OBJ: 240. ANS: OBJ: 241. ANS: OBJ: 242. ANS: OBJ: 243. ANS: OBJ: 244. ANS: OBJ: 245. ANS: OBJ: 246. ANS: OBJ: 247. ANS: OBJ: 248. ANS: OBJ: 249. ANS: OBJ: 250. ANS: OBJ: 1 C 1 A 1 D 1 B 1 C 1 D 1 B 2 B 2 C 2 B 2 A 2 A 3 D 3 STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: PTS: STA: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.6.A.6 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 1 DIF: 5.2.B.3 | 5.4.B.1 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4 I REF: 4