Phenylalanine substitution
Preparation of 2-Hydroxy-3-Phenylpropanoic Acid
Background
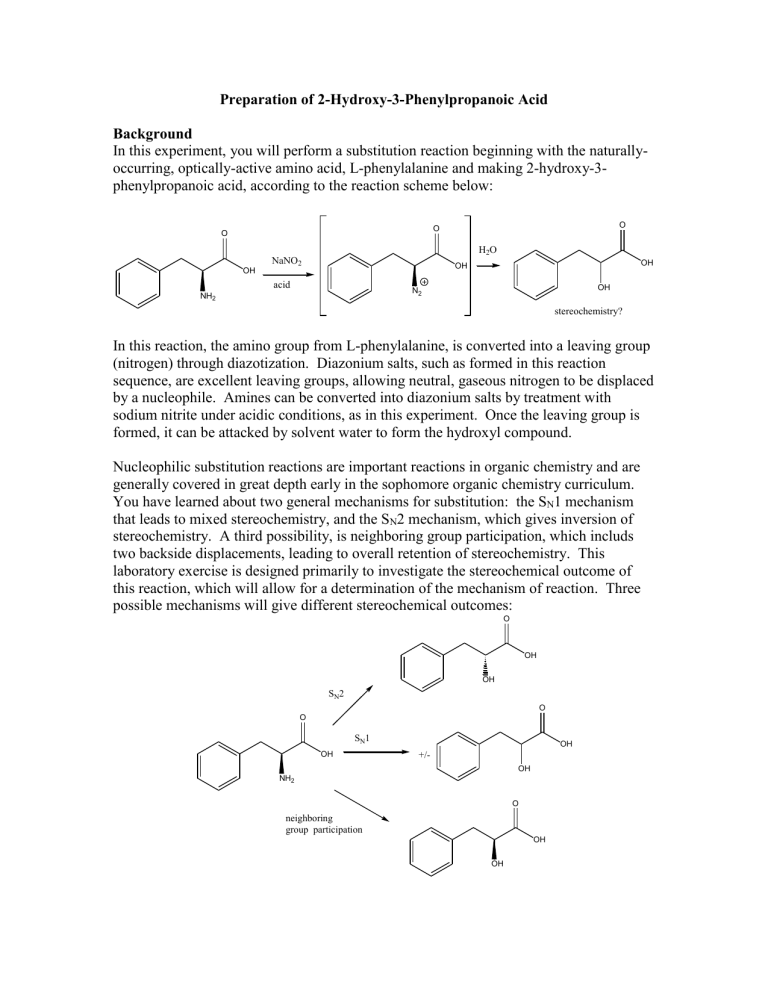
In this experiment, you will perform a substitution reaction beginning with the naturallyoccurring, optically-active amino acid, L-phenylalanine and making 2-hydroxy-3phenylpropanoic acid, according to the reaction scheme below:
O
O
O
H
2
O
NaNO
2
OH
OH
OH acid
OH
N
2
NH
2 stereochemistry?
In this reaction, the amino group from L-phenylalanine, is converted into a leaving group
(nitrogen) through diazotization. Diazonium salts, such as formed in this reaction sequence, are excellent leaving groups, allowing neutral, gaseous nitrogen to be displaced by a nucleophile. Amines can be converted into diazonium salts by treatment with sodium nitrite under acidic conditions, as in this experiment. Once the leaving group is formed, it can be attacked by solvent water to form the hydroxyl compound.
Nucleophilic substitution reactions are important reactions in organic chemistry and are generally covered in great depth early in the sophomore organic chemistry curriculum.
You have learned about two general mechanisms for substitution: the S
N
1 mechanism that leads to mixed stereochemistry, and the S
N
2 mechanism, which gives inversion of stereochemistry. A third possibility, is neighboring group participation, which includs two backside displacements, leading to overall retention of stereochemistry. This laboratory exercise is designed primarily to investigate the stereochemical outcome of this reaction, which will allow for a determination of the mechanism of reaction. Three possible mechanisms will give different stereochemical outcomes:
O
OH
OH
NH
2
S
N
2
O
OH
S
N
1
+/-
O
OH
O
OH neighboring group participation
OH
OH
Verification of the stereochemical outcome of the reaction is readily available by measuring the optical rotation of the product and comparing it to the known literature value.
Procedure:
Week 1. To a 25-mL Erlenmeyer flask containing 1.65 g (10 mmol) L-phenylalanine and a magnetic stir bar, add 10 mL of 1M H
2
SO
4
(caution: corrosive). Magnetically stir the solution at room temperature until homogeneous. Cool the solution to 3-5˚C in an icewater bath. Monitor the temperature with a thermometer clamped in place such that the magnetic stir bar does not hit the thermometer. Once the solution is cool, add 5 mL of a
3.0 M NaNO
2
(caution: strong oxidizing agent) solution dropwise via a disposable pipette. The rate of the addition should be slow enough to maintain the reaction temperature below 5 ˚C and minimize the formation of noxious brown nitrogen oxide fumes. If a significant amount of brown fumes form, slow down the rate of addition.
The addition should take about 45 minutes. During the addition, note bubbles of N
2
gas forming. Once the addition is complete, remove the ice-water bath and allow the reaction to stir at room temperature until the end of the lab period. Lightly cork the Erlenmeyer to allow gas to escape, and leave the reaction in your drawer until the next lab period.
Weeks 2-3. Cool the solution to 0-5 ˚C to maximize yield, and isolate the solid product by vacuum filtration. Wash the crystals by slurrying in 5 mL of ice-cold water in the
Büchner funnel with the vacuum released. Remove the wash solvent by vacuum filtration. Pull air through the crystals until they are mainly dry. Spread out the crystals on a watch glass to dry further. Analyze the product by melting point, 1 H NMR (acetoned
6
), IR, and specific rotation (c = 1.4, acetone).
Discussion Guidelines:
Before writing your discussion, think through the typical types of physical observations to record in a synthetic experiment. Observations should be linked to explanations—for example, consider the solubility of reactants and products. Why is the starting Lphenylalanine soluble in the reaction mixture but the product is not? Discuss the similarities and differences between the two structures in your answer.
Include comments on the “success” of the reaction based on experimental data. How high yielding was the reaction? How could it be improved in future reactions? Did you form the product that you intended to form? Does the proton NMR support this product?
(List all coupling constants and explain the splitting.)
Now to the main point: what is the stereochemistry of the product? What data support your conclusions? What is the enantiomeric excess of the reaction? Propose a mechanism for its formation. Explain how other mechanisms are excluded based on data.






![【我們是你的百姓】 [ We are Your people ] 新歌頌揚377 我們屬於祢都](http://s2.studylib.net/store/data/005298903_1-fa3ea08f8bad91a00d5f15d00abd2df9-300x300.png)