BIO 330 Cell Biology Spring 2011 Lecture Outline Macromolecules
advertisement
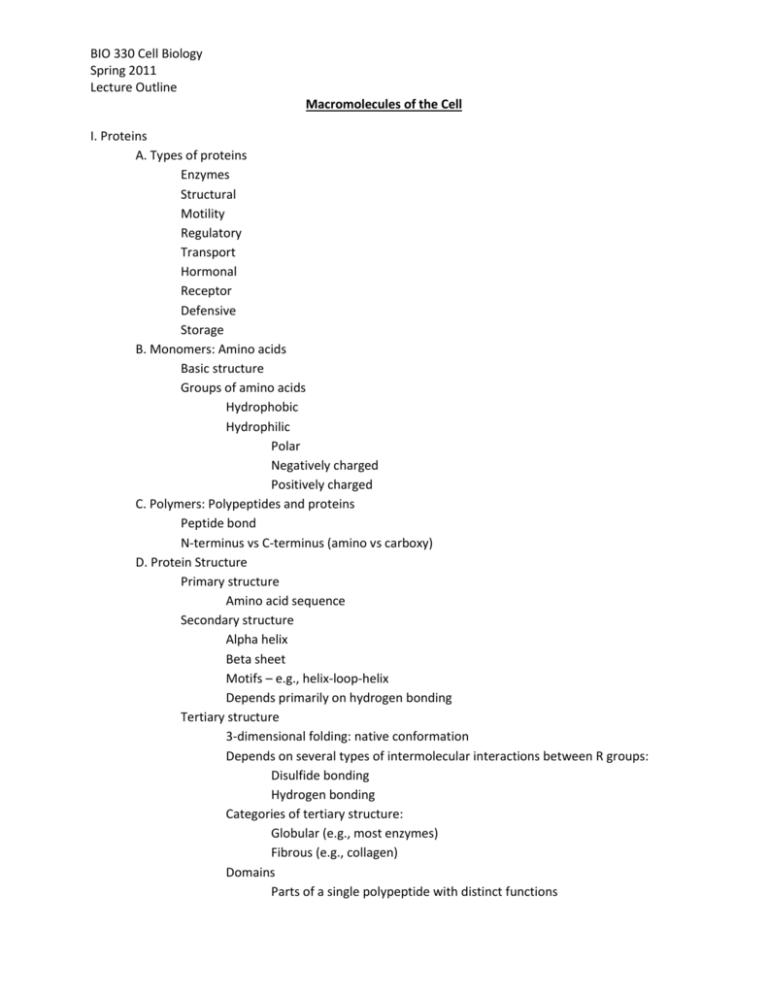
BIO 330 Cell Biology Spring 2011 Lecture Outline Macromolecules of the Cell I. Proteins A. Types of proteins Enzymes Structural Motility Regulatory Transport Hormonal Receptor Defensive Storage B. Monomers: Amino acids Basic structure Groups of amino acids Hydrophobic Hydrophilic Polar Negatively charged Positively charged C. Polymers: Polypeptides and proteins Peptide bond N-terminus vs C-terminus (amino vs carboxy) D. Protein Structure Primary structure Amino acid sequence Secondary structure Alpha helix Beta sheet Motifs – e.g., helix-loop-helix Depends primarily on hydrogen bonding Tertiary structure 3-dimensional folding: native conformation Depends on several types of intermolecular interactions between R groups: Disulfide bonding Hydrogen bonding Categories of tertiary structure: Globular (e.g., most enzymes) Fibrous (e.g., collagen) Domains Parts of a single polypeptide with distinct functions BIO 330 Cell Biology Spring 2011 Lecture Outline Quaternary structure Multiple polypeptides joining together to form one functional protein Multiprotein complex several proteins joined together, all involved in a single multi-step process – e.g., pyruvate dehydrogenase complex II. Nucleic Acids A. Types of nucleic acids DNA – deoxyribonucleic acid RNA – ribonucleic acid (ATP and GTP) B. Monomers: Nucleotides Purines: cytosine, thymine, uracil Pyrimidines: adenine, guanine Nucleotide = nucleoside + phosphate Nucleoside = sugar + base ATP = adenosine triphosphate = sugar + adenine + 3 phosphates C. Polymers: DNA and RNA Phosphodiester bonds Directional Nucleic acids are built from nucleoside triphosphates Provide energy needed to build the molecules III. Carbohydrates (Polysaccharides) A. Types of carbohydrates Simple sugars – monosaccharides E.g., glucose, galactose, fructose Disaccharides E.g., sucrose, lactose, maltose Oligosaccharides E.g., short (branching) polymers attached to cell surface Polysaccharides E.g., starch, cellulose, glycogen B. Monomers: Monosaccharides Sugar: an aldehyde or ketone with two or more hydroxyl groups Aldosugars vs ketosugars Named according to # of carbons Triose, tetrose, pentose, hexose, heptose D-Glucose: C6H12O6 (aldohexose) Ring vs linear forms Hydroxyl on C1 can be oriented “up or down” BIO 330 Cell Biology Spring 2011 Lecture Outline Orientation changes the nature of glycosidic bond Alpha vs beta C. Polymers: Storage and Structure Storage polysaccharides Starch and glycogen Alpha glycosidic bonds Branched or unbranched Starch – amylase vs amylopectin Structural polysaccharide Cellulose Beta glycosidic bonds Indigestible to mammals Bacterial cell walls Glucosamine derivatives N-acetylglucosamine (GlcNAc) N-acetylmuramic acid (MurNAc) Chitin GlcNAc only D. Three dimensional structure of polysaccharides Glycogen & starch Loosely coiled helices Cellulose Rigid linear rods Aggregate into microfibrils IV. Lipids A. Types of lipids *Common characteristic is hydrophobic nature Functional categories Energy storage Membrane structure Specific biological functions Chemical categories Fatty acids Tricylglycerols (triglyerides) Phospholipids Glycolipids Steroids Terpenes B. Fatty acids Long unbranched hydrocarbon chain with COOH at one end BIO 330 Cell Biology Spring 2011 Lecture Outline Amphipathic Even # of carbon atoms (12-20) Synthesis involves adding 2-C groups Saturated vs unsaturated vs trans fats C. Triacylglycerols Glycerol + 3 fatty acids linked by ester bonds via condensation reactions Fatty acid chains within a triglyceride are not necessarily identical Energy storage is primary function “Oil” = liquid triglyceride (plants) “Fat” = solid triglyceride (animals) D. Phospholipids Similar to triglycerides, with a polar group substituted for one fatty acid chain Amphipathic – form membranes Two types Phosphoglycerides Phosphatidic acid – precursor to many membrane molecules Two fatty acyls plus phosphate group attached to glycerol Phosphoglycerides have an alcohol attached to phosphate Alcohol: serine, ethanolamine, choline, inositol Sphingolipids Sphingosine (an amine alcohol) Ceramide = sphingosine + fatty acid “Head groups” E. Glycolipids Derivatives of sphingosine containing carbohydrate instead of lipid R group Amphipathic Usually in outer leaflet of plasma membrane F. Steroids Derivative of 4-ringed hydrocarbon Cholesterol – amphipathic Steroid hormones Estrogen, testosterone, cortisol, aldosterone G. Terpenes (isoprenoids) Derivative of isoprene – a 5-C compound Diverse biological functions Vitamin A Carotenoid pigments Doichols Coenzyme Q