carbon quiz - SemOneAPBioFinalExamReview
advertisement

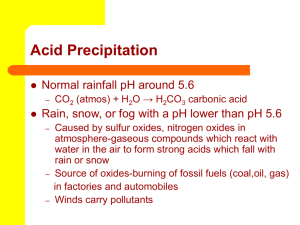
1. How many bonds can be formed to one carbon atom? a. 1 b. 2 c. 3 d. 4 2. What type of bond is most commonly formed with carbon atoms? a. Covalent and polar bonds b. Ionic and polar bonds c. Covalent and non-polar bonds d. Ionic and non-polar 3. A carbon atom has _______ electons and ________ valence electrons a. 4, 0 b. 6, 2 c. 4, 6 d. 6, 4 4. The ability to form ____________ that have ___________ allows carbon to be able to form many different bonds with many different elements a. Long chains, complex structure b. Short chains, complex structure c. Long chains, straight chains d. Medium chains, crazy chains 5. How do isomers promote carbon molecule diversity a. They allow for the differential use of single, double, and triple bonds b. They allow for different chemical properties in compounds while using the same elements. c. They make cool shapes d. They allow for longer carbon chains 6. Because of its ideal size, carbon molecules are able to bond with? a. Gases that want to gain electrons b. Metals that want to give up electrons c. Both A & C d. None of the above Answers: d, a, d, a, b, c