Honors 2- wave calculations
advertisement
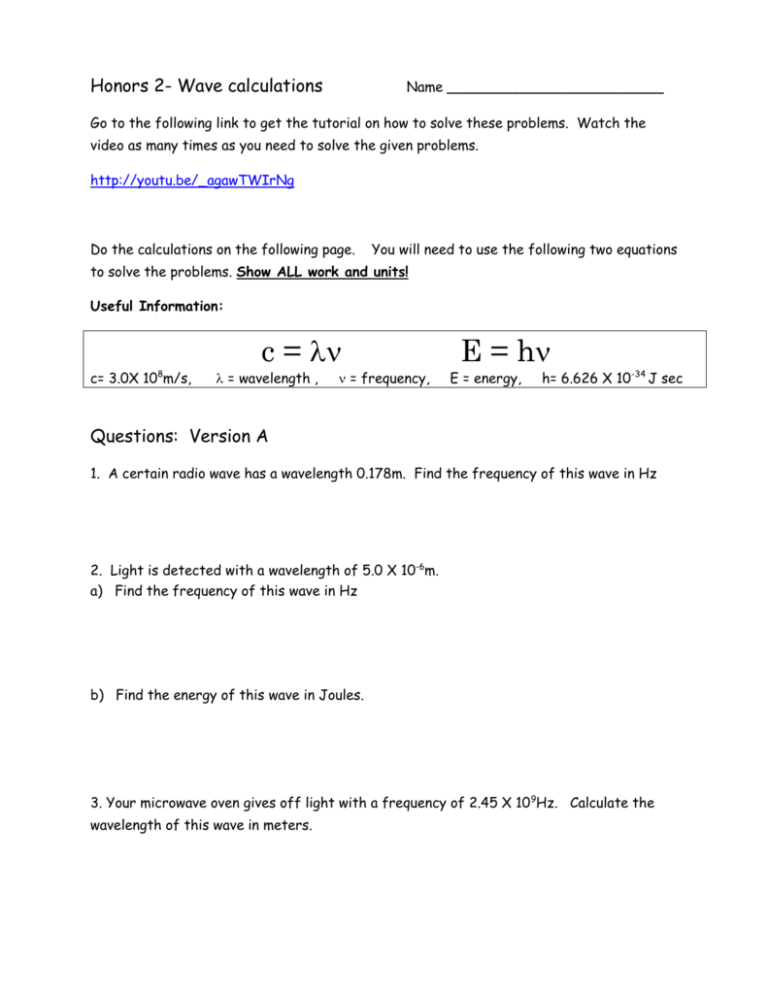
Honors 2- Wave calculations Name _________________________ Go to the following link to get the tutorial on how to solve these problems. Watch the video as many times as you need to solve the given problems. http://youtu.be/_agawTWIrNg Do the calculations on the following page. You will need to use the following two equations to solve the problems. Show ALL work and units! Useful Information: 8 c= 3.0X 10 m/s, c = = wavelength , = frequency, E = h E = energy, h= 6.626 X 10-34 J sec Questions: Version A 1. A certain radio wave has a wavelength 0.178m. Find the frequency of this wave in Hz 2. Light is detected with a wavelength of 5.0 X 10-6m. a) Find the frequency of this wave in Hz b) Find the energy of this wave in Joules. 3. Your microwave oven gives off light with a frequency of 2.45 X 109Hz. Calculate the wavelength of this wave in meters. Version A cont. 4. A wave on the border between microwave and infrared has a frequency of 2.0x1012 Hz. a) Calculate the wavelength of this wave in meters. b) Find the energy of this wave in Joules 5. Given the following three wavelengths, calculate the energy for the one that represents the highest energy. 3.37m 4.56 X 10-3m 2.68 X 10-9m 6. The energy of a given photon (packet) of light is 4.4 X 10-15 J, what type of light does this represent? (Hint – you need to find its wavelength first) Wavelength range for light: Radio waves: 103 - 1m Micro waves: 10-1 – 10-3 m Infrared waves: 10-4 – 10-7 m Visible waves: 7X10-7 – 4X10-7 m Ultraviolet waves: 10-8 – 10-9 m X-Rays: 10-10 – 10-12 m Gamma Rays: less than 10-12 Honors 2- Wave calculations Name ________________________ Go to the following link to get the tutorial on how to solve these problems. Watch the video as many times as you need to solve the given problems. http://youtu.be/_agawTWIrNg Do the calculations on the following page. You will need to use the following two equations to solve the problems. Show ALL work and units! Useful Information: 8 c= 3.0X 10 m/s, c = = wavelength , = frequency, E = h E = energy, h= 6.626 X 10-34 J sec Questions: Version B 1. A certain radio wave has a wavelength 1.32m. Find the frequency of this wave in Hz 2. Light is detected with a wavelength of 6.0 X 10-5m. a) Find the frequency of this wave in Hz b) Find the energy of this radio wave in Joules. 3. Light observed by a space telescope in the gamma region of the spectrum has a frequency of 4.1 X 1020Hz. Calculate the wavelength of this wave in meters. Version B cont. 4. Light with a frequency of 7.26 X 1014Hz lies in the violet region of the visible spectrum. a) Calculate the wavelength of this wave in meters. b) Find the energy of this wave in Joules 5. Given the following three wavelengths, calculate the energy for the one that represents the lowest energy. 3.37m 4.56 X 10-3m 2.68 X 10-9m 6. The energy of a given photon (packet) of light is 4.4 X 10-19 J, what type of light does this represent? (Hint – you need to find its wavelength first) Wavelength range for light: Radio waves: 103 - 1m Micro waves: 10-1 – 10-3 m Infrared waves: 10-4 – 10-7 m Visible waves: 7X10-7 – 4X10-7 m Ultraviolet waves: 10-8 – 10-9 m X-Rays: 10-10 – 10-12 m Gamma Rays: less than 10-12