DENSITY of SOLIDS and LIQUIDS LAB
advertisement

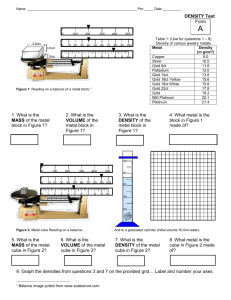
NAME: DENSITY of SOLIDS and LIQUIDS LAB WEEK I and WEEK II NOTE: WEEK I write-up will include all sections of the formal lab report. WEEK II write-up will only include data, graphs, calculations and post lab for WEEK II stations. PRELAB Read the lab and answer the following questions on a separate sheet of paper. Use complete sentences or show mathematical calculations showing work, units, and significant figures. 1. Find the equations that you would use to calculate the volume of a cube, a rectangular solid, a cylinder, and a sphere (hint-consult your planner or math text). 2. True or False – A ton of steel will have a larger density than 1 steel nail. 3. Read the volume on each of the graduated cylinder pictures shown in the background section and then determine the volume of the object that was submerged in the cylinder to make the water level rise. Report your answer to the proper number of decimal places/ significant figures. Label them Vcylinder and Vring. 4. Use the terms inversely or directly to complete the following: Mass and density are __________________proportional. Volume and density are __________________proportional. 5. Research the following substances: sintra, PVC, PP, acrylic, and HDPE. Record their full names, chemical formulas, and density values (or range of density values). What do the substances have in common? 6. Why are the theoretical density values of brass, bronze, and steel reported as a range of densities? How can you categorize these substances? (use terms from your chapter one notes) OBJECTIVE 1. To find the density of a metal cube, a rectangular solid, and a liquid sample and to use the density to identify the substance. 2. To use water displacement to determine the volume of an irregular shaped object and to use that volume to calculate density. 3. To graphically analyze mass and volume to determine the density of a sample of brass. 4. To use density to determine the % composition of pennies. 5. To determine the thickness of a piece of aluminum metal. 6. To analyze data using average, average deviation, and percent error calculations. BACKGROUND Matter can be described qualitatively through observation of intensive or extensive physical or chemical properties. This lab will explore the extensive properties of mass and volume and their relationship to the intensive property, density. Recall that mass is a measure of how much matter is in a substance. The standard unit for measuring mass is the kilogram; however, our lab balances are calibrated using grams. Volume is a measurement of the amount of space occupied NAME: by a sample of matter. Volume is measured in units of cm3 or ml. When mass is divided by volume, the result is the density of the substance. DENSITY = MASS VOLUME The resulting units of density are g/cm3 or g/ml. The obtained density value may be used to identify an unknown sample by comparing it to a chart of known (theoretical) density values. You could find such a chart in your textbook or in the CRC Handbook of Chemistry and Physics. Possible identities of substances used in this lab include: aluminum, zinc, copper, cadmium, tin, lead, bronze, brass, steel, PP, Sintra, PVC, acrylic, HDPE, ethanol, ethylene glycol, corn oil, glycerol, or water. This lab will employ two techniques to determine the volume of objects. One technique involves measurements of the dimensions of the object and using mathematical relationships to find the volume of regular shaped objects. This method assumes that the object is solid and free of air space. If, however, the solid is irregularly shaped or contains air space between particles, it is necessary to perform water displacement to find the volume. This method begins with a known volume of water in a graduated cylinder. Then the object is placed into the water and the new volume is recorded. The difference in the recorded water volumes must equal the volume of the object submerged in the water. In order for water displacement to work, the object must be completely submerged in the water and must not be soluble or able to chemically react with the water. Here are two sample graduated cylinder before and after the object is submerged. The volume of the water should be read to 1 decimal place (1 digit beyond the markings on the cylinder). The last digit is an estimated digit which adds some amount of uncertainty to the volume reading. This uncertainty may lead to errors in your density calculations. Luckily, determining the mass of each sample is quite easy. You will use the electronic balance and record the masses to the second decimal place. MATERIALS Electronic balance Weighing dish Calipers Ruler NAME: 100 ml graduated cylinder (plastic, so that it does not shatter when the sample is introduced) Pipette(s) Cubic metal samples Rectangular solid samples Irregular shaped solids samples Pennies Aluminum foil Unknown liquid samples NAME: PROCEDURES The following parts may be completed in any order. WEEK I PART I: CUBIC SOLIDS 1. Obtain a metal cube and make qualitative observations. 2. Find the mass of your cube. 3. Determine the average length of a side of the cube. 4. Return the cube to its storage box. 5. Perform calculations of volume, density, and percent error. Identify the metal sample and determine the % error of your result. PART II: RECTANGULAR SOLIDS 1. Obtain a rectangular solid and record its ID number as well as qualitative observations. 2. Find the mass of the solid. 3. Determine the length, width, and height of the solid. 4. Return the rectangular solid to its storage box. 5. Perform calculations of volume, density, and percent error. Identify the rectangular solid sample and determine the % error of your result. PART III: IRREGULAR SHAPED OBJECT 1. Obtain and irregular shaped metal object and record qualitative observations. 2. Record the mass of the object. 3. Determine the volume of the object through water displacement. Fill a 100 ml graduated cylinder to approximately the 50 ml mark. Record the exact volume to 1 decimal place. Carefully place the metal sample into the cylinder and record the new volume of the water. 4. Dry your metal sample and return it to its storage box. 5. Share your data with another group that used the same metal sample. 6. Perform calculations of volume, density, percent error, average deviation, and a precision check. Identify the irregular solid sample and determine the % error of your result. WEEK II PART I: DENSITY of BRASS 1. Obtain five brass cylinders and record qualitative observations. 2. Record the mass of each cylinder. 3. Record the length and diameter of each cylinder using the Vernier calipers. 4. Return the cylinders to their storage box. 5. Perform calculations to determine the volume of each. NAME: 6. Create a graph of mass vs. volume for the five cylinders. Include two titles, axes labels and units, data points connected by a line and show a slope calculation after determine the line of best fit through the points. The slope represents the density of brass. 7. Calculate % error. 8. Given the density of copper and zinc, determine the % of each element combined to make the alloy, brass. PART V: % COMPOSITION of PENNIES 1. Obtain 10 pennies making sure that they are all post 1982. 2. Record the mass of the 10 pennies all at once. Determine the average mass of one penny. 3. Use water displacement to find the volume of all 10 pennies together. Determine the average volume of one penny. 4. Calculate the density of the pennies using the mass of all 10/ volume of all 10 and also using the average mass of 1 penny/ average volume of 1 penny. 5. Determine the % of the penny (post 1982) that is actually copper and zinc given the fact that the inner core of the penny is zinc. PART VI: LIQUID DENSITIES 1. Select one of the four mystery liquids to identify. Record its letter and qualitative observations. 2. Weigh three empty pipets. Find the average mass of an empty pipet. 3. Use the pipet in the container of liquid and draw up 1.0 ml of solution to the line right below the bulb of the pipet. Quickly release the pressure on the bulb and invert the pipet so that the liquid flows into the bulb end. Carefully clean the exterior of the pipet. Find the mass of the pipet and liquid by placing the filled pipet in a weighing dish (that has been tared) on the electronic balance. Repeat finding the mass three times with the same liquid. 4. Calculate the average mass of the liquid, the average density, compare the density to the theoretical values and determine the identity of the mystery liquid. Report your percent error, average deviation, and precision check. PART VII: THICKNESS of ALUMINUM 1. Obtain a piece of aluminum foil. 2. Write a procedure that will allow you to determine the thickness of the piece of foil. Your procedure should involve density and should not use calipers. 3. Carry out the procedure and report your results in a data table that you design. 4. Consider averaging your data with other groups to validate your results. DATA WEEK I PART I: CUBIC SOLIDS NAME: QL observations of the metal: Calculation (show only when necessary) Value and Unit Mass of cube Length of side Volume of cube Density (experimental) Possible identity of metal Theoretical density % error PART II: RECTANGULAR SOLIDS ID # QL observations of the rectangular solid: Calculation (show only when necessary) Value and Unit Mass of solid Length Width Height Volume of rectangular solid Density (experimental) Possible identity of solid Theoretical density % error PART III: IRREGULAR SHAPED OBJECT QL observations of the metal: Calculation (show only when necessary) Mass of irregular object Final volume of water after object is submerged Value and Unit NAME: Initial volume of water Total volume of water displaced = volume of the irregular object Density (experimental) Density data from other groups: Initial each data set Possible identity of metal Group 1 Group 2 Group 3 Theoretical density Average density: Compare 4 trials % error Compare average to theoretical Average deviation Compare 4 trials Precision check Compare 4 trials WEEK II PART IV: DENSITY of BRASS QL observations of the cylinders: Cylinder # 1 2 3 4 5 Attach a full sheet graph of mass vs. volume. Density of brass from graph: mass Slope calculation: length Radius volume NAME: % error (given average theoretical density of 8.00 g/ml) % copper and % zinc in the brass sample. Show a weighted average calculation. PART V: % COMPOSITION of PENNIES Calculation (only show when necessary) Value and unit Mass of all 10 pennies at once Volume of 10 pennies at once Density using mass and volume of 10 penny data Average mass of 1 penny Average volume of 1 penny Density using average mass and volume of 1 penny Theoretical density of a post 1982 penny % error of penny density % of copper and % zinc in the penny (show work using a weighted average) PART VI: LIQUID DENSITIES Liquid letter ID: QL observations of liquid sample: Mass of empty pipet Average mass of empty pipet Mass of pipet and liquid Mass of liquid Volume of liquid inside pipet Density of liquid Average density of liquid Possible identity of liquid Theoretical density of liquid Percent error Calculations (show when necessary) 1. 2. 3. Value and unit NAME: Average deviation Precision check PART VII: THICKNESS of ALUMINUM You design the data table. The final copy should be typed. POST LAB QUESTIONS 1. 2. 3. 4. 5. WEEK I A cylinder of lead has a radius of 2.6 cm and a height of 12 cm. What is the mass of this piece of lead? A cube of aluminum has a mass of 25.50 grams. Determine the dimensions of the cube. A rectangular block has dimensions: 4.5 cm x 1.7 cm x 7.8 cm. The mass of the block is 312 grams. Based upon this, will this block float or sink in water? A cylindrical tin can has a height of 22.00 cm, an external radius of 5.00 cm and an internal radius of 4.99 cm (assume the tin has the same thickness at the sides and on its base and assume an open container). Given the density of tin = 2.8 g/cm 3, determine the mass of the empty tin can. If the can is filled with water, calculate the mass that would appear when the filled can is placed on the electronic balance. The density of petroleum oil is less than the density of sea water. Explain how this fact will help in an oil spill cleanup. POST LAB QUESTIONS WEEK II 1. An alloy of bronze was made by combining 88 % copper and the rest is composed of tin. Determine the density of the bronze sample using a weighted average. 2. Would it be possible to calculate the volume of a sample of calcium metal using water displacement? Explain. 3. Why did the pennies all have to be post 1982? Would the density have been higher or lower given pre-1982 pennies? 4. Compare the density of pennies calculated with one penny versus the density calculated with all ten pennies. Explain your findings. Why did the procedure call for you to find the mass and volume of 10 pennies at a time instead of 1 at a time? NAME: 5. Given the % of copper that you found in the post 1982 pennies and given your total mass of pennies, what is the true value of the copper in the ten pennies? Show a calculation. (hint – find the current scrap value of copper). 6. When reporting density of gases, what is the most appropriate unit? 7. The Hindenberg Zepplin airship was filled with hydrogen gas. Given the volume 7,062,000 ft 3, determine the mass of hydrogen gas that would be needed to fill the balloon. The balloon was originally engineered to be filled with He. What is the mass of He gas required to fill the balloon? Why was the balloon filled with hydrogen instead of He?