CE 428 Carbon Adsorp..
advertisement

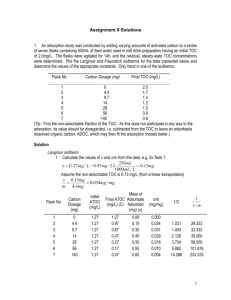
CE 428 Water and Wastewater Treatment Design Carbon Adsorption Dr. S. K. Ong _________________ - accumulation of a substance (_____________) at the interface between two phases. The phase at which the accumulation occurs (solid or even liquid) is the ___________________. ______________________ – transfer of a substance from one phase into another phase. ________________________ – used when both phenomena are occurring or cannot be distinguished Adsorption is used in water and wastewater treatment for the removal of a wide range of organic compounds, objectionable odor and color, and inorganic compounds. Typical adsorbent used is activated carbon. Activated carbon comes in two forms: _________________________. Granular activated carbon (GAC) Common sizes are US standard mesh of 12 x 40 (______________ mm) and 8 x 30 (____________ mm). Uniformity coefficient is usually _______________. Apparent density = ___________________ kg/m3 (dry activated carbon per unit volume of activated carbon, including volume of voids between grains). Particle density (wetted in water) = __________________________ kg/m3 Surface areas – ____________________________ m2/g Powdered activated carbon (PAC) – commercially available with 65 to 90 % passing through 325 mesh (_________________ m) sieve. – Surface areas – ___________________________ m2/g GAC and PAC are made from wood, peat, lignite, bituminous coal, and coconut shells. The process involves carbonization or pyrolysis (conversion of the raw materials to char - done in the absence of air at temperature < 700o C) followed by activation or oxidation (to develop the internal pores and surface characteristics – use of oxidizing gases such as steam and CO2 between 800 – 900o C). Adsorptive Capacity of activated carbon or any adsorbent is described using the adsorption isotherm. The isotherm describes the relationship between the concentration of adsorbate accumulated on the adsorbent (mass sorbed per unit mass of adsorbent) and the equilibrium concentration of the dissolved adsorbent. Adsorption Isotherms can be constructed using batch experiments: - use several containers with each container having a fixed amount of GAC (assume M gms) - different concentrations of the target adsorbate, Co but the same volume (V) is added - the containers are capped and shaked until steady state conditions are achieved (usually 24 hours) - the final liquid concentration is measured C The mass sorbed per unit mass of adsorbent, qe, is given by: Plot qe vs C Common isotherm models used: Freundlich model (equation 15.42 in your book, page 487) where K – sorption coefficient n – constant If n > 1, favorable sorption n < 1 unfavorable sorption n = 1, linear sorption isotherm (special case q e = KC). Both K and n can be found by taking the log qe vs log C. Langmuir Isotherm (equation 15.41) where Qo = maximum mass sorbed per unit mass K = constant DESIGN OF ACTIVATED CARBON COLUMNS - many approaches Irrational Approach –based on hydraulic flow rate and empty bed contact time. In a design, the information needed are: the minimum contact time needed and the carbon needed to provide the necessary removal for a given volume of water treated. The following design information are needed: _____________________, Q (both average and maximum) ________________________ (SLR): flow rate (Q)/column area (A) ____________________________ (EBCT) = Depth of carbon bed / (Q/A) Where Q = flow rate A = area of the column GAC Depth – selected based on required breakthrough Properties SLR (m3/m2/hr) EBCT (mins) GAC depth (m) Typical Range , can be as high as 4 hours for high concentrations Type of GAC – GAC made from different materials have different sorption capacities and characteristics GAC Usage rate or carbon usage rate (CUR) - indicates the mass of carbon required per unit volume of water treated. CUR = mass of activated carbon in column/volume of water treated to breakthrough, V B This is probably the most difficult to estimate without doing batch adsorption studies or pilot column studies. Based on Batch Adsorption Studies Isotherms can be used to obtain a rough estimate of activated carbon loading and bed life. Where Co C1 (qe)o Y = influent concentration = average effluent concentration for entire column run = mass adsorbed (mg/g) when C = Co = bed life (volume of water that can be treated per unit volume of carbon) = units [(mg/g GAC (g/L)/ (mg/L)] GAC = apparent density of GAC Example: Estimate the bed life and carbon usage rate for a GAC adsorber that is to remove 10 g/L of bromoform from solution, GAC = 500 g/L 1. Freundlich isotherm constants for bromoform are K = 20 and 1/n = 0.52 2. Using the Freundlich equation qe = KCe1/n (qe)o = 20 (0.01)0.52 = ______________ mg/g 3. Assuming C1 = 0, gives Y = (qe)o GAC /(Co – C1) = (1.82 500/0.01) = ____________ L H2O/L GAC = bed life 4. CUR = (Co – C1)/(qe)o = 0.01/1.82 = ___________________ g GAC/L H2O Limitations for a given breakthrough concentration, the entire GAC bed adsorber may not be in equilibrium with the breakthrough concentration the approach does not take into consideration competition amongst adsorbates Design Based on Column Studies - use bench-scale column tests to obtain data that can be used to estimate the performance of large carbon adsorbers. - breakthrough curve is obtained by plotting the normalized concentrations (C/C o) as the y-axis and time on the x-axis. - book has two methods - ________________________ - need a single breakthrough curve - bed depth service time (_________) approach – needs at least three columns and breakthrough curves - will provide a simpler method: Kinetic Approach Need a breakthrough curve and the curve is modeled using: C Co where 1 1 e K1 q o M C o V ) Q C = effluent solute concentration Co = influent solute concentration K1 = rate constant qo = maximum solid phase concentration of the sorbed solute M = mass of sorbent in test V = throughput volume Q = flow rate C K q M K C V Rearranging we have ln o 1 1 o 1 o C Q Q This is a straight line plot of ln(C/Co -1) vs V With slope = -K1Co/Q and intercept = K1qoM/Q Co is known, Q is known, M is known, K1 and qo can be found. Use new Q and C desired and determine M needed. Note that there could be scale up problems with this method. ln (Co/C -1) C/Co Volume (V) Typical Carbon Adsorbers Volume V

















![Stress Test 18 text for 3rd draft proposal[1]](http://s3.studylib.net/store/data/007159148_1-fe8823e3344c65821251ca9551e982d4-300x300.png)

