Fluid Properties
advertisement
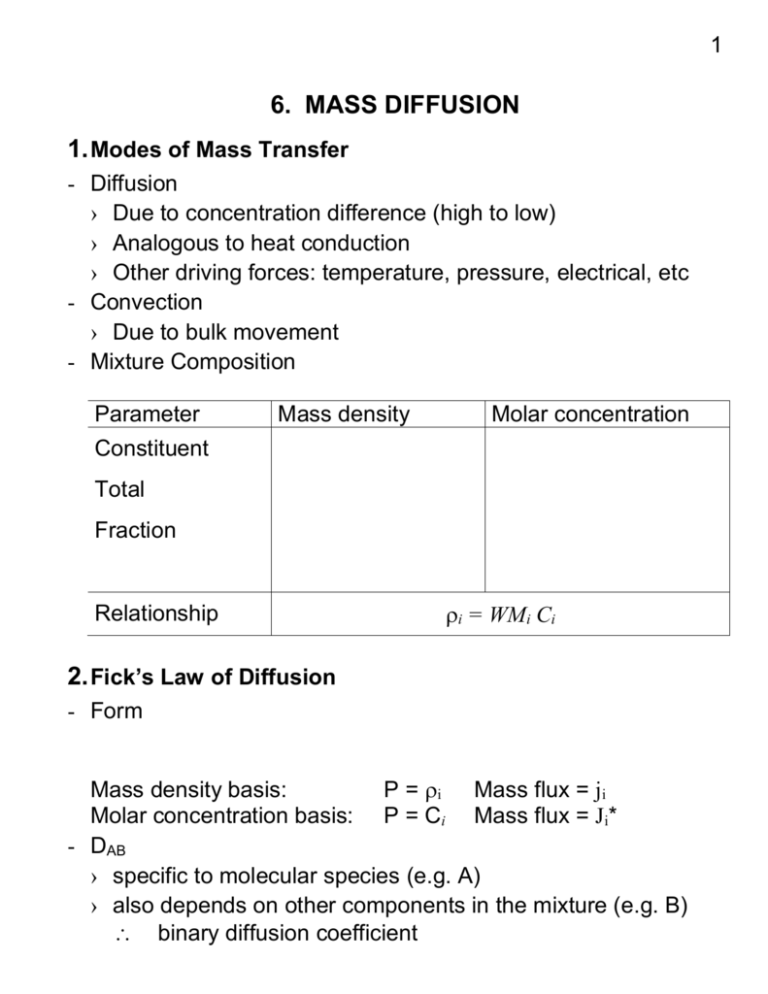
1 6. MASS DIFFUSION 1. Modes of Mass Transfer - Diffusion › Due to concentration difference (high to low) › Analogous to heat conduction › Other driving forces: temperature, pressure, electrical, etc - Convection › Due to bulk movement - Mixture Composition Parameter Mass density Molar concentration Constituent Total Fraction Relationship i = WMi Ci 2. Fick’s Law of Diffusion - Form Mass density basis: P = i Mass flux = ji Molar concentration basis: P = Ci Mass flux = Ji* - DAB › specific to molecular species (e.g. A) › also depends on other components in the mixture (e.g. B) binary diffusion coefficient 2 › For a binary mixture in 1D systems d dm A j A , kg / m 2 / s D AB A D AB dx dx dC A dx J *A , kmol / m 2 / s D AB CD AB A dx dx - Reference Frame The fluxes jA and JA* are written wrt a reference frame that is moving at a mass (or molar) average velocity of the mixture Need to develop absolute fluxes n"A or N "A that are relative to a fixed coordinate system n"A A v A N "A C A v A › Relationships v mass average velocity 1 ( A v A B v B ) m A v A mB v B v* molar average velocity 1 (C A v A C B v B ) x A v A x B v B C Redefine the relative fluxes: j A A (v A v ) A v A A v n"A A v J "A C A (v A v * ) C A v A C A v * N "A C A v * For a binary mixture: J *A J *B 0 j A jB 0 For a stationary medium: v 0 j A n"A * N "A v* 0 J A the mass and heat transfer analogy is complete 3 3. Mass Conservation Law - Mass Conservation in a CV - General Mass Diffusion Equation For a homogeneous, stationary, binary medium: › Rectangular coordinates: D A D A D A n A x AB x y AB y z AB z A t › Cylindrical coordinates: › Spherical coordinates: Some boundary conditions: › constant concentration at the surface: x A (0, t ) x A, s › impermeable surface: x CD AB A 0 x x 0 For convection-diffusion systems: Pv x Pv y Pv z x y z P G x y z x y z t where: P = I or Ci n"A or N "A include both diffusion and convection A C A n"A n A N "A N A t t 4 4. Mass Diffusion in Solids (without chemical reaction) - Some Examples › Semi-permeable membranes » Packaging » Hemodialysis » Gas separation (e.g. He from natural gas) › Movement of chemical through soil › Drying solids › Extraction of metals from ores - 1D, Steady-State, Stationary, Homogeneous, Non-reacting Governing equations: Boundary conditions: Solve for concentration profiles through the medium: m A (m A, s 2 m A, s1 ) x m A, s1 L The rate of mass diffusion: m A, s 2 m A, s1 (m A, s1 m A, s 2 ) " n A j A ( why ?) D AB L L / D AB In similar way, we can get: C ( x A, s1 x A, s 2 ) N "A J *A L / D AB See Table 14.1 for other solutions for mass diffusion Note: for an ideal gas: Ci pi / T , C PT / T and xi pi / PT 5 › Sample 1: Hemodialysis in an artificial kidney. Artificial kidney uses a membrane to separate blood on one side and dialyzing fluid on other side as shown below. Dialyzing fluid has solutes such as K+ and Cl- at noramal physiological levels but initially no urea or uric acid. The membrane is 0.025 mm thick and the total area is 2.0 m2. The concentration of urea in the blood is 0.20 g urea/L of blood. Blood Membrane Dialyzing fluid a) b) Estimate the total mass transfer coefficient, assuming kblood = 1.25 x 10-5 m/s, kdial. fluid = 3.33 x 10-5 m/s, and DAB = 2.20 x 10-5 m2/s Estimate the rate of urea removal from the blood. 6 5. Evaporation in a Column › pure liquid A in a container is open to atmosphere at the top › A is volatile, and evaporates into air (component B) › air moving across top of the container What are the concentration profiles and the rate of mass transfer in the headspace? N " N "A N B" ? N "A Rearrange it, realizing for ideal gases, x A p A / PT : CD AB dx A CD AB dp A N "A 1 x A dx PT p A dx Assuming steady-state conditions, N "A constant . dp A d 1 0 dx PT p A dx with boundary conditions: p A x 0 p A, o p A x L p A, L Note that p A, o Hx A, o from Henry’s law 7 Solving for it leads to: x/L PT p A PT p A, L 1 x A 1 x A, L or PT p A, o PT p A, o 1 x A, o 1 x A, o Because x A xB 1, we can get: x/L x/L x B x B, L x B, o x B, o The rate of mass diffusion is: CD AB dx A CD AB 1 x A, L D AB PT PT PA, L N "A ln ln 1 x A dx L 1 x RTL P P A, o A, o T Sample 2. Estimate the evaporation rate of water into dry air at 20 C and 1 atm. The liquid H2O is contained in a beaker and the distance between the liquid surface and the top of the beaker is 10 cm. C 2.5kPa DH2O-air = 2.5 x 10-5 m2/s, p 20 A, H O 2 8 6. Mass Diffusion with Chemical Reactions › Consider the stationary media (Component B is fixed) › Component A is diffusing through B and reacting › 1D, steady-state condition Gov. eq.: with BC: If the reaction rate is N A k1C A , the general solution is: C A ( x) C1e mx C 2 e mx , where m k1 / D AB Solve for the boundary conditions: C A, o Example 14.5 C A ( x) 2mx e m( 2 L x ) e mx e 1 The rate of mass diffusion is: dC A N "A D AB dx x 0 x 0 9 7. Transient Diffusion Taking an 1D, stationary medium as an example, the governing equation is: 2 2C A C A T T D AB t x 2 t x 2 if defining the dimensionless groups for mass transfer similar to those used in heat conduction: D t Fom AB2 Fo t2 Lc Lc h L Bi hL Bim m k D AB we can use all the heat transfer solutions for mass transfer problems (both analytical solutions and charts)! Sample 3. A large sheet of material 40 mm thick contains dissolved hydrogen having a uniform concentration of 3 kmole/m3. The sheet is exposed to a fluid stream that causes the concentration of the dissolved hydrogen to be reduced suddenly to zero at both surface, and maintained thereafter. If DH2-sheet = 9 x 10-7 m2/s, how much time is required to bring the density of dissolved hydrogen to a value of 1.2 kmole/m3 at the center of the sheet?










