Water Potential
advertisement

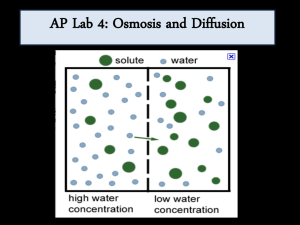
Water Potential (the Greek letter psi, pronounced "sigh") Plants must balance the intake of water with water loss. Osmosis is the passive movement of water across a cell membrane. There are openings between adjacent plant cell walls called plasmodesmata (which rhymes with stomata), so osmosis is not impeded by cell walls. FYI: The biopolymer/carbohydrate/polysaccharide, amylopectin (or simply "pectin") is a sticky substance that kind of glues plant cells together. You can buy this stuff in a refined form in stores. It is used to make jelly gel. Back to water potential: Animal cells lack cell walls, and when placed in a hypotonic solution, water will enter until the cell expands to the point of lysing (bursting). Some unicellular animal-like organisms (protozoan = "first animal") have a subcellular contractile vacuole for releasing the excess water. Plant cells have a rigid cell wall, so they can handle the internal pressure. In fact, they depend on this internal pressure. The internal pressure of plant cells resulting from osmosis is called turgor pressure. Tissues under pressure are turgid. When turgor pressure is too low, owing to moisture stress, the cells and tissues become "deflated" or flaccid. When moisture loss is severe, the plasma membrane separates from the cell wall in a process called plasmolysis. There is a concept that is unique to plants called Water Potential, . Water moves in plants by osmosis from where is higher to where it is lower. is a function of two factors; the solute potential, s, plus the pressure potential, p. So, = S + p. Solute potential is at a maximum at zero (distilled water), whereas pressure potential can be negative (vacuum), zero (one atmosphere), or positive (greater than zero). So distilled water at normal atmospheric pressure has a water potential of zero ( = 0). As solutes are added and their concentration increases, S becomes increasingly negative. Pressure potential (p) is a physical force exerted on the cell wall. As turgor pressure increases, the value for p increases proportionally. Water potential is measured in megapascals (MPa). To get a handle on values of pressure in MPa's, consider that a car tire's pressure is about 0.2 MPa. The pressure of water in your home's plumbing system is about 0.25 MPa. By comparison, most plant cells carry out their various and miraculous functions at about 1.0 MPa! This is equal to about 10X normal atmospheric pressure. So why don't plants explode when you poke a hole in them? It is for the same reason that you can't hear plants "breathe" - the pressure is confined to millions and millions of individual cells which do not release their pressure all at once. I hope the above information helps you understand and appreciate plants a little bit more. The plants provide us with all we need to live and they do so under intense pressure. Thanks to Neil Campbell (RIP) for some of the examples in the above paragraph (Campbell and Reese, "Biology", 7th Ed). Our lab with potato tissue is difficult to reconcile without a basic understanding of water potential, because there is no sucrose in the tuber tissue, only starch. However, both solutes affect in pretty much the same way. With a basic understanding of water potential, we can determine the sucrose concentration that is equivalent to the starch concentration within the tissues.