Formulas from Combustion Analysis
advertisement

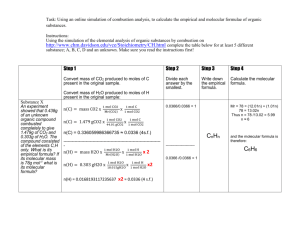
Formulas from Combustion Analysis The reaction of the organic compound with excess oxygen produces carbon dioxide and water. Conservation of mass requires that all the carbon from the organic material become carbon dioxide. Therefore the mass of carbon in the carbon dioxide is the same as the mass of carbon in the organic compound. Similarly, the mass of hydrogen in the water must be the mass of the hydrogen in the organic compound. Conservation of mass also requires that the mass of the compound be the sum of the mass of all the elements in the compound. Therefore, if the organic compound contains oxygen, the mass of oxygen can be determined by subtracting the mass of hydrogen and carbon from the mass of the entire compound. Example Problem: What is the empirical formula of a hydrocarbon that produces 2.703 g CO2 and 1.108 g H2O when combusted? Note: Since this is a hydrocarbon, the only elements it contains are carbon and hydrogen. Solution: The empirical formula will be the simplest mol ratio of these elements. nC = 2.703 g CO2 x nH 1 mol CO2 x 1 mol C = 0.06142 mol C 44.01 g 1 mol CO2 = 1.108 g H2O x 1 mol H2O x 2 mol H = 0.1230 mol H 18.01 g 1 mol H2O To get the simplest ratio, divide the moles of each substance by the lowest mol value. 0.06142 mol C = 1 0.06142 0.1230 mol H = 2.003 0.06142 Round to the nearest whole number to use in the formula. Therefore the empirical formula for this compound is CH2. Question: What is the empirical formula of a substance containing carbon, hydrogen, and oxygen if 1.000 g of substance produces 1.467 g CO2 and 0.6003 g H2O upon combustion? Solution strategy: The mass of carbon dioxide and water can be used to find the masses and moles of carbon and hydrogen. The mass of oxygen can be determined from the mass of the original substance. Because of conservation of mass: Mass (g) substance = mass carbon + mass hydrogen + mass oxygen