Physics 2010
advertisement

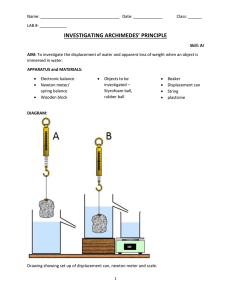
5.1 Physics 2010 Archimedes' Principle Experiment 5 The density of an object is defined as its mass m divided by its volume V. m V (1) (The Greek letter , rho, is pronounced "row".) In this experiment, you will determine the density of some irregularly shaped solids whose masses can be determined easily by weighing, and whose volumes are hard to measure directly. The simplest way to find V is to lower the solid into a calibrated beaker of water. The rise in the level of the water tells you the volume of the object, as shown below. When you use this method, you will discover that it is not very accurate. V=volume of solid A much more accurate method is based on Archimedes' Principle which states: When a solid of volume V is immersed in a fluid, it experiences a buoyant force FB equal to the weight mg of fluid which it displaces: that is, FB mg f Vg (2) where f is the density of the fluid. If f is known and FB is measured, then from Eqn (2) one can calculate the volume V of the solid. In this experiment, you will immerse the solid in water, whose density is known to be water 1000 . gram / cm3 1000 . kg / liter = 1000 kg / m3 . To find the buoyant force FB , you will weigh a solid object, first out of the water and then suspended in the water, as shown below. In the first case, the weight of the object is supported by the string alone: therefore, the tension T in the supporting string is T = mg. In the second case, the weight is born by the string and the buoyant force together, and the tension is T mg FB . The difference of these two tensions is the required force FB. 5.2 reading = map FB T = mg - FB reading = m T = mg - FB T = mg mg Forces on solid when immersed. A small complication is that most scales (including those in this lab) are calibrated in units of mass, not in units of force. The reading of the scale is not the tension in the string, but the tension divided by g. Therefore, when you weight the block out of the water and the tension is mg, the reading is just m, the mass of the block. (This is obviously convenient and is why the scale is calibrated in this way.) When you weigh the block under water, the tension (or apparent weight) is T mg FB . Thus, the reading on the scale is the apparent mass map, map mg FB g (3) You can solve (3) for FB to give FB ( m map ) g . (4) Combining (2) and (4), we find that water V g ( m map ) g , or V m map water . (5) Notice how the factors of g cancel. The expression (5) for V is based on Archimedes' Principle. Thus, by comparing your value obtained from (5) with your earlier rough measurement of V, you can test 02/06/16 5.3 Archimedes' principle. However, since the value obtained from (5) is much more accurate, it is this value that you will use in the rest of the experiment. Knowing the mass and volume of your block, you can calculate its density. By comparing your value with the known densities of the metals available in this laboratory, you can identify what the metal is. You will then repeat this exercise for a second metal block. Once you know the volume of a solid, you can use the relation (5) to find the density of a fluid other than water. In Part II of this experiment, you will immerse both of your blocks in anti-freeze. Eqn (5) still holds, except that the denominator is now af , the density of anti-freeze, and m ap is the apparent mass obtained by weighing the block in the anti-freeze. Solving (5) for af , we find that af m map V . (6) Since you can do this with both blocks, you can get two answers for af . Comparing them gives a sensitive test of the accuracy of your measurements. If you have been careful, your two values will agree reasonably well to four significant figures. Procedure Part I. Densities of Metals In this experiment it will be convenient to use cgs units (centimeter-gram-second). 1. There is an assortment of irregularly shaped blocks of aluminum, brass, steel, and lead. Pick two blocks, of different metals, weigh both of them, and record their masses. With care, you can find the masses within one or two hundredths of a gram on a four-arm balance, but you must be careful that the balance is zeroed properly; that is, that it reads zero when nothing is on it. (Your instructor can help you to adjust this if necessary.) You might weigh one block on two or three different balances to find out how reliable they are. 2. Put some water into a calibrated beaker (the kind with a handle, which has 5 ml graduations, 1 ml = 1 milliliter = 1cm3) and lower one of your blocks into the water. Record the volumes to the nearest 1 ml, without and with the block, and calculate V by subtraction. The beaker has 5ml divisions, but you can estimate volumes to the nearest ml. Do this two or three times for the same block, with different amounts of water in the beaker. State your best estimate for V and its approximate uncertainty. 3. To find the volume of each of your two blocks using Archimedes' principle, you need to weigh each block while it hangs immersed in water. The four-arm balance has an adjustable platform on which the beaker of water can rest, as shown in the diagram below. (Only the beakers without handles will fit). Put this platform above the pan of the 02/06/16 5.4 balance, and place the beaker on it. Suspend the first block from the appropriate hook, making sure that it is totally immersed. Be sure that the block is not touching the side of the beaker and that the beaker is not rubbing against the bars that support the balance pan. Also, check that no bubbles are adhering to the block, especially in the hole for the string. Adjust the length of the supporting string and the amount of water, if necessary. Now find the apparent mass map of the first block immersed in water. Use Eqn (5) and your measurements of m and map to calculate the volume V of your first block. Repeat this determination of map and V for the second block. Comment in your lab book about whether the two values of V for the first block obtained in steps 2 and 3 agree. 4-arm balance platform pan m V of each block. Given the following know approximate densities, identify the composition of your blocks. Is this identification consistent with the appearance of the blocks? (Brass is yellowish-gold, lead is a dull gray and soft, aluminum is silvery and light, and steel is silvery-gray and considerably heavier than aluminum.) 4. Using your more accurate values of V for the two blocks, calculate the density metal aluminum brass steel lead 02/06/16 density (gram/cm3) 2.7 8.5 7.8 11.3 5.5 (Note: These densities are approximate. Both brass and steel are alloys or mixtures of various pure metals, and their densities vary according to their composition. Although aluminum and lead are, in principle, pure metals, the samples in the laboratory almost certainly contain impurities which change their densities slightly.) Part II. Density of Anti-Freeze Using the known volumes of your blocks, you can now find the density of the anti-freeze available in the bottles near the sink. Caution! Anti-freeze is poisonous. It is also moderately expensive. When you have finished, pour it back into the bottle, not down the drain. Never pour anything toxic down a drain. It all eventually goes into the drinking water supply and purification is never perfect! Empty your beaker of water, dry it, and fill it with the anti-freeze. Now find the apparent masses of your two blocks when immersed in the anti-freeze. Use Eqn (6) and your data to get two values for the density af of the anti-freeze. Comment on how well your two values of af agree. If you have been careful, the percent discrepancy may be as small as 0.1%. Questions 1. Suppose there was a tiny bubble of air clinging to the block when you weighed it in water. If the volume of the bubble was Vbub , then the volume V computed from Eqn (5) would have been Vblock Vbub . If Vbub were 1mm3, what would have been your computed density of your first block? Note that 1mm = 0.1 cm, so 1mm3 = 0.001 cm3. What is the percent change in computed density? Is it a significant effect? 2. In part I, you weighed the blocks out of the water to find their masses. In truth, they were immersed in a fluid - namely the air - and there was a small buoyant force on them. Therefore, the values that you took to be their true masses were really their apparent masses in air. Using the known volume V of your first block, calculate how big a difference this makes to your measurement of m. The density of air is about 1 kg/m3 or 10-3 gram/cm3 in Boulder. Is the effect significant? 02/06/16 5.6 Physics 2010 Pre Lab Questions Experiment 5 1. What is Archimedes Principle? 2. A rock is thrown in a beaker of antifreeze and it sinks to the bottom. Is the buoyant force on the rock greater than, less than, or equal to the weight of the rock? Explain your answer. 3. A chunk of metal is held stationary, suspended by a string and the tension in the string is 19.6 N. What is the mass of the chunk of metal? 4. If the metal in question 3 is brass, use the known density of brass (given in the lab writeup) to find the volume of the chunk of metal. 5. A mass m is weighed while immersed in water and suspended by a string attached to a balance. The balance shows the apparent mass map of the mass when it under water. How is the volume V of the rock related to the density of water, the mass of the rock, and the apparent mass read on the balance? (Also, what is the density of water?) 6. Dry Lab. 02/06/16