UV Radiation and the Atmosphere

UV Radiation and the Atmosphere
UV radiation and the atmosphere
Mehul Malik
Τitle:
Author:
Basic idea: Ultraviolet light is invisible to us but can be detected using certain materials sensitive to it.
Jargon: Ultraviolet, Spectrum, Fluorescence
Deeper explanation:
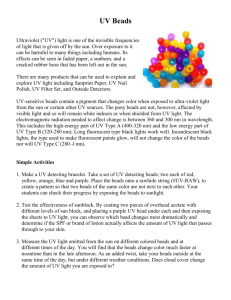
Ultraviolet light can’t be seen by the human eye but is a part of sunlight. It can be harmful to us if we are exposed to it (sunburn!) but it is mostly absorbed by the atmosphere. The presence of UV light in sunlight can be shown by using fluorescing UV-sensitive beads. Sunblock can absorb UV light and hence protects us against its harmful effects.
Importance in Science:
Harmful effects: Overexposure to certain kinds of UV light causes DNA damage which can lead to skin cancer.
Security: Credit cards and drivers’ licenses often include a UV watermark than can only be seen under a UV light.
Astronomy: Very hot stars emit a lot of UV light and hence can be seen by telescopes outside the Earth’s atmosphere.
Medicine: UV light is used to treat certain skin conditions psoriasis and eczema.
Everyday examples:
Sunburn
Credit Cards and drivers’ licenses
Extended Discussion and Demonstration
EM Spectrum and UV light:
The light that we can with our eyes is only a small part of the different wavelengths that are possible in nature. All the possible wavelengths together are called the
“Electromagnetic (EM) Spectrum.” The term electromagnetic is used because light is an electromagnetic wave, consisting of oscillating electric and magnetic fields. The visible part of the EM spectrum ranges in wavelength from 400 to 700 nm. The color red has a wavelength of 700 nm while blue has a wavelength of 400 nm. Any wavelength longer than 700 nm is called infrared (IR) and any wavelength shorter than 400 nm is called ultraviolet (UV).
UV light ranges from about a 100 to 400 nm in wavelength, and is further categorized as
UV A, B and C (another way of classifying UV light is Near, Middle, Far, Vacuum and
Extreme). The smaller the wavelength of UV light, the more energy it carries and the
more dangerous it is. UVB and UVC are both extremely harmful to living things and are mostly blocked by the Ozone layer in the atmosphere. 98.7 percent of UV light that reaches the Earth’s surface is UVA. The light from a Black Light consists of UVA radiation.
Excessive exposure to all forms of ultraviolet radiation can cause many harmful effects in our skin.
Overexposure to UVB can cause sunburn and damage DNA which can lead to skin cancer.
UVA, B and C can all damage collagen fibers and hence cause our skin to age faster. Both
UVA and B destroy vitamin A in skin. It is important that a screen protect against all different forms of UV.
While overexposure of UV light has many harmful effects, it is also beneficial. UVB light induces the production of Vitamin D in skin, which is essential for healthy bone growth.
Equipment:
1 Black Light
Regular flashlight
Assorted UV-sensitive beads
Pipe cleaners
Sunblock
Cream cheese
Transparency film or acetate sheets
Spatula or butter knife
Fig. 1. The EM Spectrum
Source: http://en.wikipedia.org/wiki/Image:Electromagnetic-Spectrum.png
UV-sensitive bead demonstration:
The presence of UV light in our atmosphere can be demonstrated using beads that are sensitive to UV light (available at www.teachersource.com
).
1.
String some beads on a few pipe cleaners in order to avoid scattering or losing the beads.
2.
If there is sunshine, let students take the beads out to a window or outdoors to see the beads change color. Tell them to see if the beads change color when placed in the shadow as well (due to scattering of UV light by the atmosphere).
3.
Turn on the black light and show how the UV beads change color strongly when placed under the black light.
4.
Apply sunblock on a transparency film and place between the black light and beads to show the absorption of UV light.
5.
Apply cream cheese on another film to show that even though it is opaque, cream cheese does not block UV light. source: http://en.wikipedia.org/wiki/Image:Solar_Spectrum.png