Exam 1
advertisement

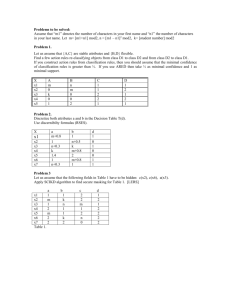
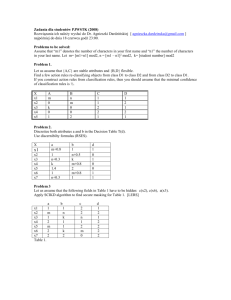
Dr. Casey Exam 1 Spring 2009 ENVS 602 Name: ______________________ Signature: ______________________ Honor Statement: By signing this exam, you indicate that you have neither given nor received aid from any unauthorized source. Answer each question in the space provided. If you need more space, use the back of the sheet. All work must be shown to receive partial credit. Useful Information [G] = KH * PG 1 atm = 101,325 Pa 1 atm = 760 mm Hg = 760 torr 1. One form of sulfur in the environment is the polyatomic ion sulfite (SO32-). When fully protonated, it is known as sulfurous acid (H2SO3). The acid dissociation constants for sulfurous acid are as follows: Ka1 = 1.72 x 10-2 Ka2 = 5.81 x 10-13 a. Write the chemical reactions that correspond to Ka1, Ka2 and 2 for the sulfite system. b. Instead of dissociation reactions, rewrite the reactions as formations (forming HSO3and H2SO3) starting from SO32-. Identify the reactions as Kf1, Kf2 and f2 and specify the values for these constants. c. Write an expression for the total SO32- in the system. Then write that same expression in a form where the concentrations are expressed in terms of parent material, constants and controlling variables. 2. Arsenic contamination is being evaluated in a stream and the lake into which it flows. Two sites are chosen from which arsenic speciation of the sediment will be evaluated. Inorganic arsenic can be found either as AsO3-3 or AsO4-3. Site Characteristics: Site 1: sandy, low organic matter content, rapidly moving stream Site 2: silty, high organic matter content, static lake bottom a. Which arsenic species is expected to dominate at each site? b. Iron oxides precipitates have been shown to adsorb (attract) inorganic arsenic. Given the following reaction, in which site is this most likely to be relevant? Fe2+(aq) Fe(O)OH(s) 3. Henry’s law assumes that gas in the atmosphere is in equilibrium with dissolved gas in the aqueous phase. For the case of CO2 in a small pond, describe conditions in which this equilibrium assumption is likely to be valid and conditions in which it will be invalid. 4. Nitrous oxide (N2O) is produced in wetlands by microbial activity and is a potent greenhouse gas in the atmosphere. The partial pressure of N2O in the atmosphere is approximately 0.032 Pa. KH = 0.025 mol/(L*atm) a. What would the concentration of N2O be in a water body that is in equilibrium with the atmosphere? b. In a wetland system, the concentration of N2O is found to be 6.2 x 10-8 M. Is this concentration in equilibrium with the atmosphere? If not, will the wetland water release N2O to the atmosphere or absorb N2O from the atmosphere? Explain your answer. 5. Graph 1 Graph 2 These graphs show the same data graphed in two different ways. The data are for a sediment toxicity test in which the toxicity is due to Pb contamination. Acid volatile sulfide levels may vary between the sites. a. From the following x-axis labels, assign the best label to these graphs. More than one label may reasonalby explain the observed patterns. Graph 1, Graph 2 or neither i. Total sediment Pb ii. Free porewater Pb iii. Sediment acid volatile sulfides iv. Sediment simultaneously extractable metal v. AVS/SEM ___________ ___________ ___________ ___________ ___________ b. Explain why the data in graph 1 are more scattered while the data in graph 2 are more clustered. c. Explain the likely role of sulfide in the toxicity of these sediments. From the toxicity data, rank the sediments in terms of highest and lowest AVS levels. 6. Data for Cu speciation in two different sites are presented below. Stream 1 is located in the mountains: pH = 7.6 [DOM] = 3.5 mg/L Total Cu2+ binding sites on the DOM: 0.24 µmol/mg DOM [Cl ] = 22 mg/L Stream 2 is located in the coastal plain: pH = 5.4 [DOM] = 21.9 mg/L Total Cu2+ binding sites on the DOM: 1.17 µmol/mg DOM [Cl ] = 4 mg/L Complexation reactions to consider: Cu2+(aq) + Cl-(aq) CuCl+(aq) log β1 = 0.30 Cu2+(aq) + 2 Cl-(aq) CuCl2 (aq) log β2 = -0.26 Cu2+(aq) + OH-(aq) CuOH+ (aq) log β3 = -7.50 Cu2+(aq) + 2 OH-(aq) CuOH2 (aq) log β4 = -0.26 Cu2+(aq) + DOM(aq) CuDOM+ (aq) log β’5 = 5.36 for Stream 1 log β’6 = 4.48 for Stream 2 a. Write the mass balance equation for Cu2+ in terms of parent material, controlling variables and constants (do not substitute in numerical values). b. Why do the DOM equilibrium constants and binding site concentrations differ between the sites? c. Without calculating α values for this system, can you determine which complexes will form to the greatest extent (chloro, hydroxy, or DOM)? On what do you base your answer? d. Again, without calculating actual values, at which site will the α values be greater for the hydoxy complexes? Why?