Bloch`s Theorem
Periodic Potential Energy of Electrons in a Crystalline Environment
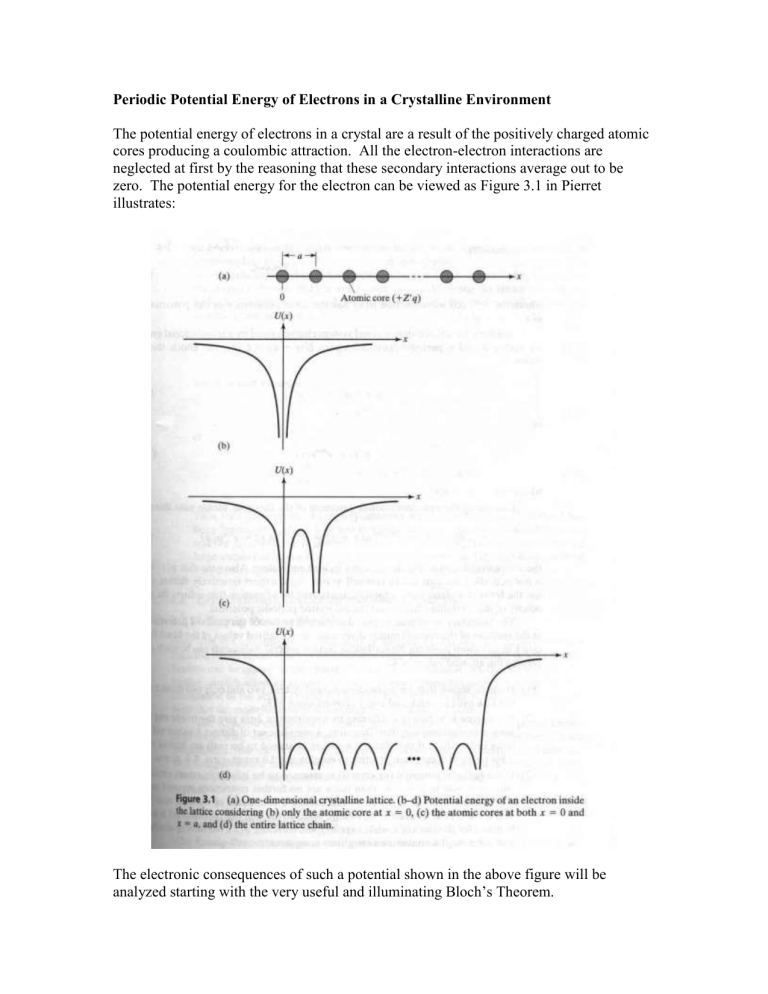
The potential energy of electrons in a crystal are a result of the positively charged atomic cores producing a coulombic attraction. All the electron-electron interactions are neglected at first by the reasoning that these secondary interactions average out to be zero. The potential energy for the electron can be viewed as Figure 3.1 in Pierret illustrates:
The electronic consequences of such a potential shown in the above figure will be analyzed starting with the very useful and illuminating Bloch’s Theorem.
Bloch’s Theorem
The solutions of the Schrodinger equation for a periodic potential must be of the form:
k
u k
e i k
r where u k
( r )=u k
( r + T ) is an amplitude function of the planewave exp(i kr ) and T is a translation vector of the crystal. The eigenfunctions of the wave equation for a periodic potential are the product of a planewave exp(i kr ) times a function u k
( r ) with the periodicity of the lattice. Wavefunctions of this form are called Bloch’s functions and they are very useful in calculations because they allow us to concentrate on only one period of the lattice to solve for the wavefunction of the electrons throughout the entire crystal.
Pierret has four general statements:
1.
It can be shown that, for a 1D system, tow and only two distinct values of k exist for each and every allowed value of energy E.
2.
For a given E, values of k differing by a reciprocal lattice vector G give rise to one and the same wavefunction solution. Therefore, a complete set of distinct kvalues will always be obtained if the allowed k-values are limited to 0 to G or equivalently – G/2 to G/2. This leads to the concept of Brillouin Zones.
3.
For an infinite crystal, k can assume a continuum of real values in the range specified in statement 2.
4.
For a finite crystal, we adopt periodic boundary conditions. This means that we construct a “ring” of N atoms such that:
k
k
x
Na
k
k
x
Na
e ikNa
k
Hence, e ikNa
1 and therefore: k
2
m
m=0,1,2,3...
Na
Kronig-Penny Model
It is extremely difficult to solve Schrodinger’s equation for the potential shown in Pierret
Figure 3.1. Therefore, the Kronig-Penny model is often used to illustrate many consequences of the periodic potential. The Kronig-Penny model make the following simplification of the potential as illustrated in the following figure of Pierret Figure 3.2:
We can now solve Schrodinger’s equation for the simplified potential shown in Fig. 3.2b.
Particle Motion and Effective Mass
When we analyze the motion of electrons in crystals, one must work with wave packets composed of a superposition of energy eigenfunctions centered around a value E o
. This is because of Heisenberg’s Uncertainty Principle which states that there is a relationship between the uncertainties in the energy and time evolution of a system:
E
t
2
Now with wave packets, one can construct a picture of charge carrier motion.
The goup velocity of a wavepacket centered around a frequencey
and wavevector k
is:
v g
k
1
E
k
If an external force is applied to the system ( i.e.
, the electron described by the wave packet), then work is done according to: dE
Fdx
Fv g dt
Also, we have:
F
d dt combining these relations together we can put it in the form:
F
m
* dv g
where m
*
1
2
E dt 1
2 k 2
The term m
*
is called the effective mass and is inversely proportional to the curvature of the E-k curves. This underscores the importance of E-k diagrams that will be used for the remainder of this course to describe the electronic properties of semiconductors.
Also note that m
*
can be both positive and negative indicating that for some situations, when an external force is applied to the system, electrons can move in a direction opposite of the direction predicted by classical physics.
Near the top or bottom of any band, the E-k curve is, to a good approximation, parabolic in nature allowing us to write:
E
E edge
const .
k
2
k edge
2 and hence it is seen that:
It is seen that m
*
is positive near the bottoms of all bands and negative near the tops of all bands. const .
m
*
Carriers and Current
One additional property of each energy band is that there are N distinct k-states in each band for a finite crystal of N atoms. If the atoms contribute 2 electrons per atom to these states, then the first two energy bands are totally filled with higher energy bands vacant.
However, temperature effects cause some electrons to be thermally excited to these higher energy bands and in turn leave a vacancy or “hole” in the lower lying energy band.
Totally empty bands will not contribute to conduction.
Totally filled bands will not contribute to conduction.
For the nearly empty band: I
3
q
L
filled v i
For the nearly filled band: I
2
q
L
filled v i but since: all _
v states i
0 we have: I
3
q
L
filled v i
q
L all _
v i states
q
L
empty v i where v i
is the velocity of the empty state.
Hence, the concept of the charge carrier denoted as a “hole” is born that is:
1.
Positively charged vacancy near the top of a band
2.
When you do the procedure –e
e you have to let m *
-m * and you have completed the conversion from the negative mass and negatively charged electron state to a postive mass and posively charged hole.
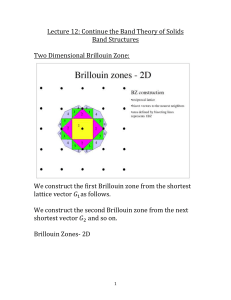
Brillouin Zones and E-k diagrams
When working with real crystals which are 3 dimensional, your crystal momentum k is now a 3D vector k . Just as in the 1D case where we can describe all distinct states using a the range 0
k
2 d
or equivalently
d
k
d
, we can restrict k-space ( i.e., reciprocal space) in 3D crystals to the Wigner-Seitz primative unit cell. This Wigner-
Seitz primative unit cell is called the the First Brillouin Zone . There exists additional
Brillouin zones, such as the second Brillouin zone and third Brillouin zone which can be carried into the first Brillouin zoned by the addition of either one or two primative reciprocal lattice basis vector respectively.
To try to plot out the energy for each k-value in the first Brillouin zone is extremely difficult, so generally the energies of the states for important directions in k-space are plotted. These important directions are from
L and
X .
The valence band is defined as the top-most band that is filled with electrons except for a few vacancies or holes. E v
is the maximum energy of this valence band. E v
always occurs at
.
The conduction band is defined as the bottom-most band that is empty except for a few thermally excited or otherwise present electrons. E c
is the minimum energy of this band which does not have to occur at
.
The bandgap is defined as E gap
=E c
-E v
and is one of the most important electronic properties of a semiconductor that effects almost every aspect of its electrical behavior as
will be seen throughout the remainder of this course. If E c
occurs at
, then the bandgap is called a direct bandgap. If E c
does not occur at
, then the bandgap is called an indirect bandgap. Direct bandgap semiconductors are used for optical applications since a high energy but very small momentum photon can efficienty excite an electron from the valence to the conduction band. Whereas for indirect bandgap materials, a photon has to be accompanied by a low energy but high momentum phonon to excite an electron from the valence to the conduction band.


![Semiconductor Theory and LEDs []](http://s2.studylib.net/store/data/005344282_1-002e940341a06a118163153cc1e4e06f-300x300.png)