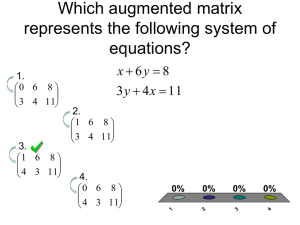
Solution of the Schrödinger Equation for an LCAO-MO
advertisement

Solution of the Schrödinger Equation for an LCAO-MO H (i ) (i ) (i ) mo mo mo mo mo (i )H (i ) (i )d (i ) (i )d mo mo mo mo mo (i ) (i )d mo mo c where ( mo mo c mo m m n n and n is the basis set n is an identical summation) m substitute: (i ) H (i ) (i )d (i ) (i )d c (i ) H (i ) c (i ) d mo m mo m m mo mo mo n mo mo n c (i ) c (i )d mo n m m m n n n c n m c (i ) H (i ) (i )d c c (i ) (i )d c n m c H c c S c m c H S m m m n n m n mo mn n n mo m n mo mn mn mo m n m mo n S (i ) (i )d mn m n m n m n mn 0 H (i ) H (i ) (i )d mn m Hamiltonian integrals Overlap integrals n are integrals over the basis set . n c m n m c H S n mn mo mn 0 is the Schrödinger Equation, integrated and reorganized in an LCAO-MO form. The problem is to solve this for c and n mo . The Variation Principle: The ground state of the problem will be the one with the lowest possible energy. For any variable in the wavefunction , the best choice of its value is / 0 . given by: mo For the LCAO-MO problem this means that: / c 0 for all n. mo n Differentiate the equation c m n m c H S n noting that mn H ,S mn mo mn mn 0 wrt c n are constants / c c c H S 0 2c H S c c S / c 0 c H S 0 for all n n m m m n m n mn m mn mo mn m mn mo mn mo m n mn m n mn mo n The SECULAR EQUATIONS For these equations to have a non-trivial solution requires: det H S 0 mn mo mn The SECULAR DETERMINANT Solving the Secular Determinant give (many) values of the molecular orbital energy a mo then substituting a mo energy into the Secular Equations gives the corresponding mo wavefunction c a n Methodology: 1) Choose a geometry for the molecule (Born Oppenheimer approx.) 2) Choose a Basis Set of N atomic orbitals n 3) Obtain values of the Hamiltonian integral 4) From where?? Set up the N x N Secular Determinant det H S 0 mn mo mn and solve for N values of 5) For each value of a c mo , i.e. mo mn and overlap integrals S a mo solve the Secular Equations for c which gives the a n mo a LCAO-MO H a n n n This effectively solves the molecular orbital Schrödinger equation for N mo.s of the molecule. mn .