CHAPTER 14. Human Effects: Air Pollution and Heat Islands
advertisement

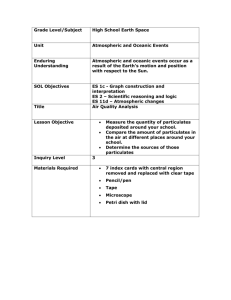
CHAPTER 14. Human Effects: Air Pollution and Heat Islands Chapter Overview: The principal types of atmospheric pollutants are discussed in this chapter. Natural and anthropogenic sources are described along with pollution effects on humans and various areas of the ecosystem. In addition, the chapter explores causes and characteristics of urban heat island. Chapter at a Glance: Most air pollutants are quickly dispersed and removed from the atmosphere so that they are rather harmless. Other pollutants may cause everything from a dull sky to death. Smog is a combination of smoke and fog. An extreme example of smog was the 1952 London air pollution episode, which left nearly 4000 people dead. Many industrial centers and large cities have air pollution problems mainly stemming from industrial processes and photochemical smog developing from a combination of automobiles and certain atmospheric conditions. Los Angeles-type smog occurs when automobile pollutants combine with dry air. Recent concerns have led to improvements in air quality, however, other negative aspects of the atmosphere-human relationship abound. • Atmospheric Pollutants - Particulates, deriving from human and natural sources, flourish throughout the atmosphere as do polluting gases. Concentrations of particulates and certain gases in urban areas invoke concerns. Pollution may be divided into either primary pollutants, those emitted directly into the atmosphere, or secondary pollutants, resulting from chemical transformations. <Web> A. Particulates - Particulates (aerosols) may be either solid or liquid materials in the air. They stem from both natural and anthropogenic sources and range in size from 0.1 to 100 µm. <Web> 1. Sources of Particulates - Natural sources of particulates include fires, volcanic eruptions, salt from breaking waves, and vegetation sources (pollen). Human sources largely involve combustion of fossil fuels in creating primary and secondary particulates. Because many particulates act as condensation nuclei, fog and the resulting smog may be problematic in large cities and industrial areas. <Web> 2. Removal of Particulates - Although particulates are small and may, therefore, remain suspended in the atmosphere for long periods, all are eventually removed in time. Gravity and precipitation are the primary mechanisms of removal. 3. Effects of Particulates - Particulates relate to factors such as reduced visibility and health concerns. Smaller particulates or PM10 particulates (particulates < 10 µm) enter the lungs and affect health. Even smaller particulates, PM2.5s, have been deemed even more dangerous. B. Carbon Oxides - Carbon dioxide plays an important role in the global energy balance but is not considered a pollutant as no direct adverse effects occur from 135 high concentrations. Carbon monoxide, however, is a colorless, odorless gas emitted naturally through volcanic eruptions, forest fires, bacterial activities, etc. In cities, unsafe levels may accumulate from incomplete combustion of gas in automobiles. Other anthropogenic sources include furnaces, home fires, and cigarette smoke. Carbon monoxide is extremely hazardous to the point of death with long-term exposure to even small concentrations. <Web> C. Sulfur Compounds - Atmospheric sulfur compounds, in gaseous and aerosol form, derive mainly from natural sources. The most important natural source is bacterial release of hydrogen sulfides but volcanic eruptions and sea spray also contribute. Anthropogenic release of sulfur dioxide (SO2) and sulfur trioxide (SO3) stems mainly from fossil fuel burning and smelting of ores. Sulfur dioxide is an irritant to human respiratory systems. Sulfur trioxide, which usually stems from reactions involving SO2, combines with water to form sulfuric acid (H2SO4). This creates acid fog and acid rain, which may cause ecosystem degradation. <Web> D. Nitrogen Oxides - Nitrogen oxides, compounds of nitrogen and oxygen, are commonly discussed as NOx. Nitric oxide (NO) forms naturally from biological processes in soil and water and anthropogenically through combustion in automobiles, industry, and electricity generation. It is broken down quickly through natural processes. Nitrogen dioxide (NO2), a toxic gas which is yellow to reddish-brown in color, contains a pungent odor and is very corrosive. It contributes to acid deposition and other secondary pollutants. Although it breaks down quickly, it varies with traffic concentrations in urban areas. Long-term exposure contributes to pulmonary health problems. E. Volatile Organic Compounds (Hydrocarbons) - Hydrocarbons, composed solely of hydrogen and carbon atoms, include methane, butane, propane, and octane in addition to other compounds. Plant and animal emissions, decomposition, and automobiles are primary hydrocarbon sources. Combined with solar radiation, these chemicals form photochemical smog. F. Photochemical Smog - Photochemical smog produces burning eyes, sore lungs, odor, and poor visibility. These smogs consist of secondary pollutants to create a Los Angeles-type smog common in many cities when smog develops in dry air. Ozone, an important contributor of photochemical smog, may cause serious physical and environmental harm. Physical ailments associated with ozone include decreased lung capacity with long-term exposure leading to permanent lung damage. <Web> • Atmospheric Controls on Air Pollution - Natural sources of aerosols usually do not create high concentrations due to release over large areas. Pollution problems exist when high concentrations occur over small spatial scales. The atmosphere contributes to pollution concentration as it is responsible for horizontal and vertical dispersion. A. Effect of Winds on Horizontal Transport - Strong winds disperse pollutants 136 through rapid transport from the high concentration source. Pollutant concentrations are inversely proportional to wind speed. Increased winds also indirectly reduce concentrations as eddies mix air vertically through forced convection. Wind direction is also important in dispersing pollutants through many directions or by concentrating pollutants in one or a few directions. B. Effect of Atmospheric Stability - Stability is also important with regard to pollution dispersion. A stable atmosphere restricts vertical motions, thereby, allowing increased pollution concentrations near the surface. Radiation induced inversions exacerbate these situations, especially during early morning hours. Subsidence inversions, usually associated with photochemical smogs, may extend for long temporal periods. Mixing depth is proportional to the inversion base. • Urban Heat Islands - Urbanized regions typically maintain higher surface temperatures than surrounding areas. These urban heat islands are usually proportional to the size of the city and the density of population. Intensity of the heat island varies spatially with highest temperatures usually located within the city core. <Web> A. Radiation Effects - Urban particulates affect the intensity of urban heat island by affecting the local radiation balance. Increased particulate concentrations absorb and scatter insolation and also increase the amount of longwave radiation release. The latter offsets the former leading to higher temperatures. Indirectly, these particulates acting as condensation nuclei relate to cloud cover and precipitation over cities leading to higher precipitation totals downwind of the city. City surfaces also contribute to urban heat island as natural vegetative surfaces are replaced by impervious ones. Heat exchange, albedo, evaporation, and radiation balances are all offset by these surfaces. B. Changes in Heat Storage - Buildings heat quickly during the day and these increased temperatures are transferred to building interiors. At night, this stored energy is released to the surface. Buildings have a much greater heat capacity than natural surfaces, which increases the amount of stored heat energy available for atmospheric warming. C. Sensible and Latent Heat Transfer - Moisture near the surface uses available energy through evaporation and transports that energy aloft as latent heat. Decreased evaporation associated with the dry surfaces of cites decreases the amount of vertical energy transport, thereby increasing the amount of surface sensible heat. 1. Urban Heat Islands and the Detection of Climate Change - Most temperature data come from urban settings. Unprecedented growth over the past century in urban areas has likely affected temperature readings. Therefore, caution should be used when examining temperature data at face value to detect climate change. Contaminated temperature data are frequently discarded or corrected for urban heat island effects. 137 Chapter Boxes: 14-1 Focus on the Environment: Air Pollution - Natural or Anthropogenic? - Natural phenomena such as El Niño events may exacerbate pollution stemming from anthropogenic sources. Such was the case in Indonesia in 1997 in which human induced fires raged. 14-2 Focus on the Environment: the Counteroffensive on Air Pollution - Although local and state laws to regulate pollution existed in the US since the late 19th century, it was not until 1963 that a national effort was initiated. The Clean Air Act sought widespread air pollution control and stimulated research and technical development. Maximum standards were set for many atmospheric aerosols through the act and its many amendments. One such amendment called for the elimination of lead in gasoline. The act also required states to establish enforcement agencies in addition to requiring automobile emission reduction devices. Because many states and urban areas failed to meet initial standards, the amendments of 1990 were established. These widespread standards call for mandatory compliance. 14-3 Focus on the Environment: Smog in Southern California - The EPA classifies Los Angeles as an extreme area of noncompliance of ozone standards. Air quality in this region is poor due to blocking mountains, sunny days, presence of a subsidence inversion, and a high concentration of automobiles. New regulations for automobile emissions, special gas pump nozzles, and new low hydrocarbon gasolines have combined to reduce the problem. Summer smogs are a common problem due to high residual primary and secondary pollutants, weak winds in the morning hours, a low morning sun angle, and the presence of fog. By late morning the development of a sea breeze helps alleviate the problem. Related Web Sites: Smog: www.smogcheck.ca.gov/smoghome.htm Acid Rain: www.epa.gov/acidrain/ardhome.html Urban Heat Island: www.climatenews.com Particulates (Aerosols): http://sasa.pmel.noaa.gov Carbon Monoxide: www.phymac.med.wayne.edu/FacultyProfile/penney/COHQ/co1.htm Air Pollution: www.epa.gov/oar Media Enrichment: ME14.1 - Weather Image - Atmospheric Pollution as Seen from the Space Shuttle ME14.2 - Weather Image - Pollution over Amazon Basin ME14.3 - Weather Image - Kuwait Oil Field Fires ME14.4 - Weather Image - Industrial Pollution in Siberia ME14.5 - Weather Image - Ozone Concentration and Air Temperature ME14.6 - Weather Image - Atlanta Urban Heat Island I, II and III Key Terms: air pollution carbon oxides acid rain 138 photochemical smog primary pollutants secondary pollutants particulates pm10 pm2.5 carbon monoxide carbon dioxide sulfur dioxide acid fog sea breeze front nitrogen oxides London-type smog nitric oxides Los Angeles-type smog nitrogen dioxide urban heat island volatile organic compounds (hydrocarbons) Review Questions: 1. Explain the distinction between primary and secondary pollutants. Primary pollutants are those emitted directly into the atmosphere. Secondary pollutants result from chemical transformations of pre-existing substances and/or pollutants. 2. What are particulates and how are they introduced into the atmosphere? Particulates may be either solid or liquid materials in air. They stem from both natural and anthropogenic sources. 3. What are the two processes most responsible for removing particulates from the atmosphere? Gravity and precipitation are the may processes of pollutant removal. 4. What are PM10 and PM2.5? Does one pose a greater health risk than the other? Particulates with a size less than 10 µm (PM10) are capable of entering the lungs. As such they pose a significant health threat. PM2.5 particulates are even smaller and are more dangerous. 5. List the most important gases that contribute to air pollution. Carbon dioxide, sulfur compounds, nitrogen oxides, volatile organic compounds (hydrocarbons) and ozone are generally the most important polluters. 6. What are the primary sources of carbon monoxide in the atmosphere? If these sources are nonanthropogenic, why is it that CO is considered a pollutant? Volcanic eruptions, forest fires, bacterial action and other natural processes and by humans though combustion. Natural sources emit far more CO than human, however, in cities, automobiles re the primary source. 7. In what way does carbon monoxide harm the human body? CO is extremely toxic. Even low levels can cause a person to immediately experience slowed reflexes, drowsiness, and a reduction or loss of consciousness. Exposure for 3 hours at 400 ppm is threatening and at 1600 ppm death occurs within an hour. Long-term exposure to low levels may lead to heart disease. 139 8. What are the primary sources of sulfur dioxide and sulfur trioxide in the atmosphere? Both stem from fossil fuel burning. Specifically sulfur trioxide usually stems from reactions involving sulfur dioxide. 9. Would a person be more likely to notice the presence of high CO or high SO2 contents in the ambient air? Carbon monoxide is colorless and odorless, so sulfur dioxide would be most noticeable. 10. Which primary pollutant is most likely to promote the formation of acid fog or acid rain? Acid fog and rain stem from sulfur trioxide, which combines with water to form sulfuric acid. 11. Why is it that nitric oxide is much less common in the atmosphere than nitrogen dioxide? Nitric oxide breaks down quickly through natural processes. Nitrogen dioxide does as well but it increases with traffic concentrations in urban areas. 12. Describe the general composition of volatile organic compounds. Hydrocarbons are composed solely of hydrogen and carbon atoms. They include methane, butane, propane, and octane in addition to other compounds. 13. How do London-type and Los Angeles-type smog differ from each other? Los Angeles-type smog is common in many cities when smog develops in dry air. It develops from photochemical reactions between hydrocarbons and sunlight. London-type smog is actually a mix of smoke and fog, typical of wet environments. 14. Which pollutant gases cause a noticeable coloration of the atmosphere? Which have a characteristic odor? Nitrogen dioxide is yellow to reddish-brown in color and contains a pungent odor. 15. Describe the various atmospheric controls that affect the concentration of air pollutants. Wind is the primary means of transporting high concentrations of pollutants from a location. As they horizontally transport pollutants the pollutants disperse. Forced convection also contributes to mixing. Wind direction is important in dispersion or by concentrating pollutants in one or a few directions. Stability is also important as this regulates vertical transport. For instance, mixing depth is proportional to inversion base associated with early morning hour smog. 16. How does the construction of buildings in cities alter the radiation exchange near the surface 140 and contribute to the urban heat island effect? Buildings heat quickly during the day and these increased temperatures are transferred to building interiors. At night, this stored energy is released to the surface. Buildings have a much greater heat capacity than natural surfaces, which increases the amount of stored heat energy available for atmospheric warming. 17. Describe the way in which heat storage in cities differs from that of surrounding rural areas. Rural areas contain natural surfaces which are permeable and typically vegetated. These surfaces allow for the evaporation of water from the surface that transports heat energy vertically. Cities, with impervious surfaces have a much greater sensible heat flux than rural areas. This results in higher temperatures for the cities. 18. What effect does urbanization have on the exchange of sensible and latent heat? Decreased evaporation rates associated with the dry surfaces of cities decreases the amount of vertical energy transport, thereby increasing the amount of surface sensible heat. 19. Does the urban heat island manifest itself equally during the day and night? Are there seasonal differences in the magnitude of the heat island effect? Stored energy is available for transfer to the lower atmosphere during the evening and nighttime, which increases nocturnal temperatures. Heating is greatest during the winter, which partially explains why heat islands are most pronounced during that season. 141