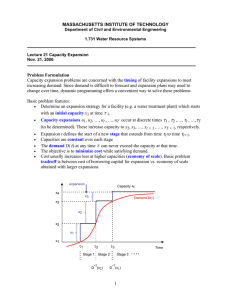
Chapter 07 FE Problem Solutions
advertisement
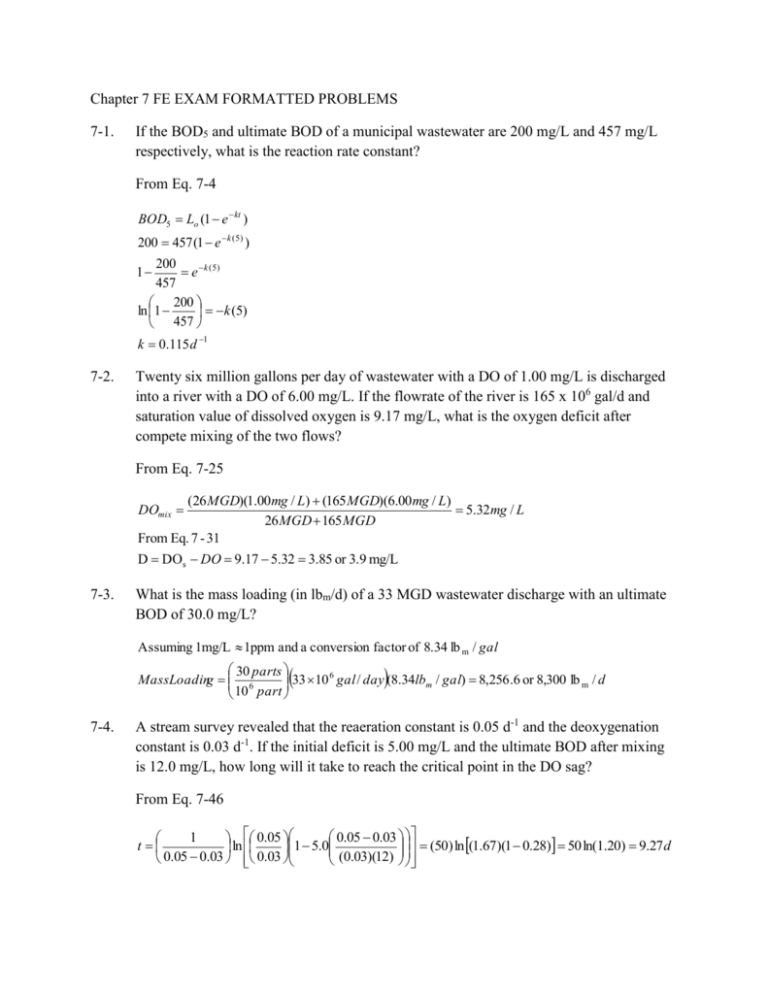
Chapter 7 FE EXAM FORMATTED PROBLEMS 7-1. If the BOD5 and ultimate BOD of a municipal wastewater are 200 mg/L and 457 mg/L respectively, what is the reaction rate constant? From Eq. 7-4 BOD5 Lo (1 e kt ) 200 457 (1 e k (5) ) 200 1 e k ( 5) 457 200 ln 1 k (5) 457 k 0.115 d 1 7-2. Twenty six million gallons per day of wastewater with a DO of 1.00 mg/L is discharged into a river with a DO of 6.00 mg/L. If the flowrate of the river is 165 x 106 gal/d and saturation value of dissolved oxygen is 9.17 mg/L, what is the oxygen deficit after compete mixing of the two flows? From Eq. 7-25 (26 MGD)(1.00 mg / L) (165 MGD)(6.00 mg / L) 5.32mg / L 26 MGD 165 MGD From Eq. 7 - 31 D DOs DO 9.17 5.32 3.85 or 3.9 mg/L DOmix 7-3. What is the mass loading (in lbm/d) of a 33 MGD wastewater discharge with an ultimate BOD of 30.0 mg/L? Assuming 1mg/L 1ppm and a conversion factor of 8.34 lb m / gal 30 parts 33 10 6 gal / day (8.34lbm / gal) 8,256 .6 or 8,300 lb m / d MassLoading 6 10 part 7-4. A stream survey revealed that the reaeration constant is 0.05 d-1 and the deoxygenation constant is 0.03 d-1. If the initial deficit is 5.00 mg/L and the ultimate BOD after mixing is 12.0 mg/L, how long will it take to reach the critical point in the DO sag? From Eq. 7-46 0.05 0.03 1 0.05 (50) ln (1.67)(1 0.28) 50 ln(1.20) 9.27 d t ln 1 5.0 0.05 0.03 0.03 (0.03)(12)