Radioactive decay chains
advertisement

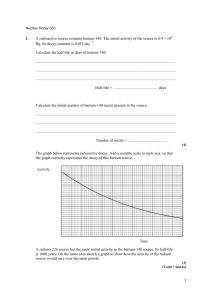
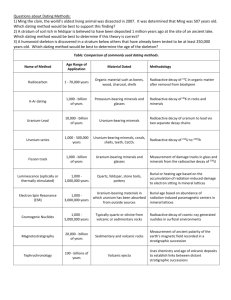
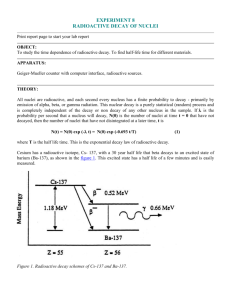
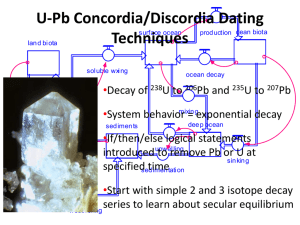
RADIOACTIVE DECAY CHAINS An unstable nucleus may emit an alpha or beta particle to form another element, or a gamma ray and become less excited nucleus. This daughter product as it is called may also be radioactive and so a whole chain of decay products can be formed. One of the most well known of these is the decay chain of uranium 238 (shown below). Isotope Uranium 238 Thorium 234 Protactinium 234 Uranium 234 Thorium 230 Radium 226 Radon 222 Polonium 218 Lead 214 Bismuth 214 Polonium 214 Lead 210 Bismuth 210 Polonium 210 Lead 206 Half life 4.5 x 109 years 24 days 1.2 minutes 2.5 x 105 years 8.0 x 104 years 1620 years 3.8 days 3.1 minutes 27 minutes 20 minutes 1.6 x 10-4 s 19 years 5.0 days 138 days stable Lead 206 is stable and is therefore the end of the decay chain. Since all the nuclei are in dynamic equilibrium in a decay series - that is there can be no build up of any particular element the rate of decay of all the components in the series must be the same in other words dN/dt for every component is the same. Therefore dN1/dt = -λ1N1 = dN2/dt = -λ2N2 = dN3/dt = -λ3N3 = dN4/dt = -λ4N4 = etc. so:One consequence of this is that the elements with the long half lives will be present in larger quantities than those with short half lives because; λ1N1 = λ2N2 etc. or λN = a constant Because N is a constant and the half life (T) of a radioactive isotope in the decay chain is proportional to 1/ we have for a decay chain Decay chains: N1/N2 = T1/T2 The number of nuclei of a particular radioactive isotope in a decay chain will be inversely proportional to the half-life of that isotope when equilibrium conditions have been reached. Example problem Find the ratio of the anmount of lead 214 to bismuth 214 in the above series. Ratio of numbers of nuclei = TPb/TBi = 27/20 = 1.35 times as much Lead 214 as there will be Bismuth 214. Notice that we have to make an adjustment if we want to deal with the relative masses of components in the series. 1 Since N = (m/M)L T1/T2 = N1/N2 = (m1/M1)/(m2/M2) = (m1/m2)x(M2/M1) Uses of radioisotopes Radioactive isotopes can be very useful. They are used in: 1. Medicine for both treatment and diagnosis 2. Archaeological and geological dating using carbon 14 or uranium 3. Fluid flow measurement - water, blood, mud, sewage etc. 4. Thickness testing of materials such as polythene 5. Radiographs of metal castings 6. Sterilisation of food and insects 7. Tracers in fertilisers used in agriculture 8. Smoke alarms in houses 9. Tracing phosphate fertilisers using phosphorus 32 10. Checking the silver content of coins 11. Atomic lights using krypton 85 12. Testing for leaks in pipes Proof of A = Ao/2n This can be proved as follows. Start with the standard radioactive decay law and take logs to the base e: A = Aoe-t ln A = ln Ao - t = ln Ao – ln(2t/T) where T is the half life. Therefore: ln A = ln[Ao/2n) where n = t/T and so A = Ao/2n 2