HW2
advertisement
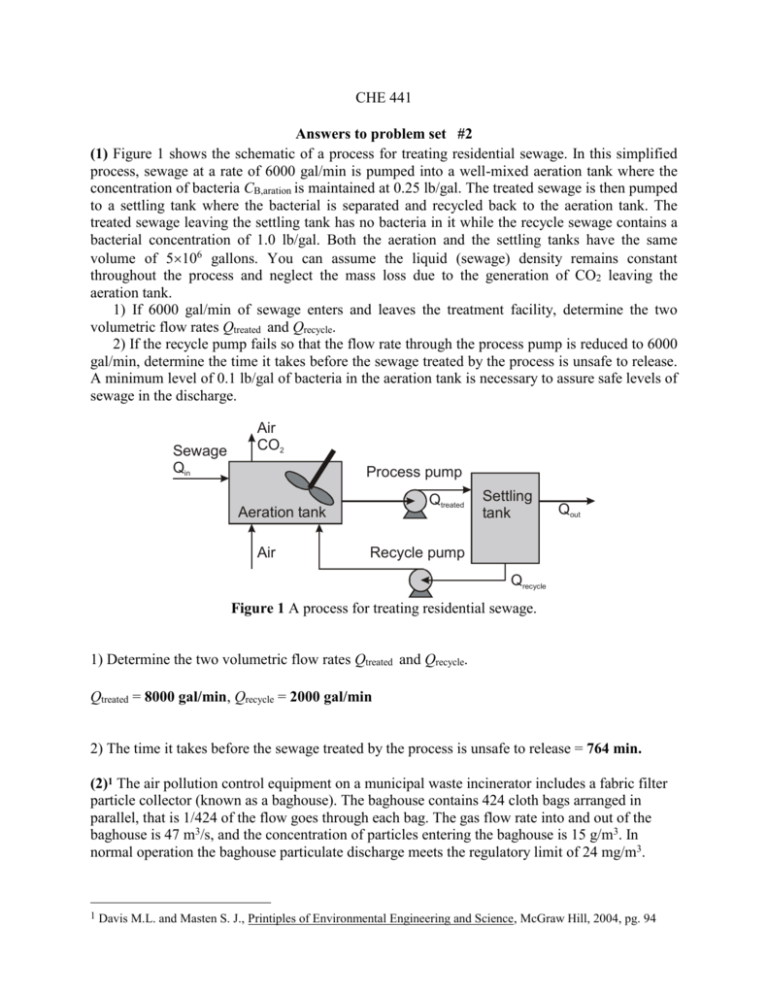
CHE 441 Answers to problem set #2 (1) Figure 1 shows the schematic of a process for treating residential sewage. In this simplified process, sewage at a rate of 6000 gal/min is pumped into a well-mixed aeration tank where the concentration of bacteria CB,aration is maintained at 0.25 lb/gal. The treated sewage is then pumped to a settling tank where the bacterial is separated and recycled back to the aeration tank. The treated sewage leaving the settling tank has no bacteria in it while the recycle sewage contains a bacterial concentration of 1.0 lb/gal. Both the aeration and the settling tanks have the same volume of 5106 gallons. You can assume the liquid (sewage) density remains constant throughout the process and neglect the mass loss due to the generation of CO2 leaving the aeration tank. 1) If 6000 gal/min of sewage enters and leaves the treatment facility, determine the two volumetric flow rates Qtreated and Qrecycle. 2) If the recycle pump fails so that the flow rate through the process pump is reduced to 6000 gal/min, determine the time it takes before the sewage treated by the process is unsafe to release. A minimum level of 0.1 lb/gal of bacteria in the aeration tank is necessary to assure safe levels of sewage in the discharge. Sewage Qin Air CO2 Process pump Qtreated Aeration tank Air Settling tank Qout Recycle pump Qrecycle Figure 1 A process for treating residential sewage. 1) Determine the two volumetric flow rates Qtreated and Qrecycle. Qtreated = 8000 gal/min, Qrecycle = 2000 gal/min 2) The time it takes before the sewage treated by the process is unsafe to release = 764 min. (2)1 The air pollution control equipment on a municipal waste incinerator includes a fabric filter particle collector (known as a baghouse). The baghouse contains 424 cloth bags arranged in parallel, that is 1/424 of the flow goes through each bag. The gas flow rate into and out of the baghouse is 47 m3/s, and the concentration of particles entering the baghouse is 15 g/m3. In normal operation the baghouse particulate discharge meets the regulatory limit of 24 mg/m3. 1 Davis M.L. and Masten S. J., Printiples of Environmental Engineering and Science, McGraw Hill, 2004, pg. 94 During preventive maintenance replacement of the bags, one bag is inadvertently not replaced, so only 423 bags are in place. Calculate the fraction of particulate matter removed and the efficiency of particulate removal when all 424 bags are in place and the emissions comply with the regulatory requirements. Estimate the mass emission rate when one of the bags is missing and recalculate the efficiency of the baghouse. Assume the efficiency for each individual bag is the same as the overall efficiency for the baghouse. Solution Efficiency of particulate removal = 99.84% Efficiency of bag house with 1 bag missing = 99.605 % (3) You want to separate all the coal particles having a diameter of 80 μm or larger from a slurry. To do this, the slurry is pumped into the bottom of the large settling tank as shown. It flows upward and flows over the top of the tank, where it is collected in a trough. If the solid coal has SG = 1.4 and the total flow rate is 250 gpm, how big should the tank be? Feed (Q) Overflow Overflow A Q/A Vt Underflow Dt = 12.447 ft (4) A gravity settling chamber consists of a horizontal rectangular duct 6 m long, 3.6 m wide, and 4 m high. The duct is used to trap sulfuric acid mist droplets entrained in an air stream. The droplets settle out as the air passes through the duct and can be assumed to behave as rigid spheres. If the air stream has a flow rate of 8.0 m3/s, what is the diameter of the largest particle that will not be trapped by the duct using quiescent model? (Acid: ρ = 1.75 g/cm3, μ = 3 cP. Air: ρ = 0.0075 g/cm3, μ = 0.02 cP.) Solution D = 0.0136 cm = 136 m (5) Your assignment is to design a cyclone to remove at least 98% of particles from a flue gas stream. The flue gas stream flow rate is 5000 m3/min. at 216oC and 1 atm. A particle rate of 3.16 lb/s enters with the flue gas. The flue gas composition and particle size distribution are listed in Table 1. The particle density is 3400 kg/m3. The maximum pressure drop allowed is 4,000 Pa. Use Prop (TK program) for viscosity and density of the flue gas. Table 1 Flue gas composition and particle size distribution Flue gas species Mole fraction dp (μm) wt % CO2 0.076 19.00 2.55 N2 0.442 41.50 3.04 SO2 0.003 49.00 1.39 H2O 0.374 64.50 9.76 CH4 0.105 83.00 24.01 Total 1.000 120.50 24.40 181.50 24.93 212.00 9.91 Calculate the size of a standard cyclone using the following procedure: 1) Assume a cyclone diameter D = 4 m. 2) Evaluate other cyclone dimensions (W, H, De, Lb, and Lc) using Figure 2, cyclone type (3) Q 3) Calculate the gas inlet velocity V = , where Q is the gas volumetric flow rate. WH L 1 4) Calculate the number of revolutions (N) in the outer vortex, N= [ Lb + c ] H 2 5) Calculate the “50% cut diameter”, dpc, defined by 1 2 9 W , where is the gas viscosity. dpc = 2 NV p g 6) Calculate the collection efficiency, i, of each particle size i = 1 1 (d pc / d pi ) 2 7) Calculate the overall efficiency, o, of the cyclone: o = i i , where i is the mass fraction of particles in the ith size range. If o < 0.98, assume another diameter and repeat step 2-7. 8) Calculate the pressure drop across the cyclone P = 8gV2 HW De2 D(m) =5 efficiency = 0.988, Dp(N/m2) = 1726.58 >> (6) A stream containing 7.5 kg/s water, 6.0 kg/s acrylonitrile, and 25 ppm NH3 is separated into an acrylonitrile layer and an aqueous layer in a decanter. A negligible amount of water is dissolved in the acrylonitrile layer, but 0.068 mass fraction acrylonitrile is found in the aqueous layer. Both the aqueous layer and the acrylonitrile layer from the decanter contain ammonia, with the concentration in the aqueous layer higher by a factor of 4.3. (a) Determine the amount (kg/s) of acrylonitrile in the aqueous layer. (b) If the aqueous layer contains 0.50 kg/s acrylonitrile, determine the concentration (ppm) of NH3 in the acrylonitrile layer. Solution (a) Determine the amount (kg/s) of acrylonitrile in the aqueous layer. 0 kg wa ter Acrylonitrile la ye r 7.5 kg wate r Aq ue ous layer 0.068 = m a /(m a + 7.5) a = 547 kg (b) If the aqueous layer contains 0.50 kg/s acrylonitrile, determine the concentration (ppm) of NH3 in the acrylonitrile layer. Ca = 8.4586 ppm (7) A well-mixed sewage lagoon (a shallow pond) is receiving 430 m3/d of sewage out of a sewer pipe. The lagoon has a surface area of 105 m2 and a depth of 1.0 m. The pollutant concentration in the raw sewage discharging into the lagoon is 180 mg/L. The organic matter (pollutant) in the sewage degrades biologically in the lagoon according to first-order kinetics. The reaction rate constant is 0.007 /d. Assuming no other water losses or gains, find the steady state concentration of the pollutant in the lagoon effluent. Solution 3 430 m /da y 5 180 mg/L Surfa ce a rea = 10 m 1.0 m 2 3 430 m /da y 68.497 mg/L (8) A dust-bearing gas stream enters a sedimentation chamber at a rate of 10 m3/s with a particle loading of 0.25 kg/m3. The volume of the sedimentation chamber is 200 m3 with a height of 2 m. If the terminal velocity of the particle is 0.05 m/s, determine the particle loading (kg/m3) of the gas stream leaving the chamber using a) Continuous quiescent sedimentation, b) Complete-mix sedimentation, c) Plug flow vertical mixing sedimentation. Solution Continuous quie sc ent sedimenta tion Ab = Volume/height = 200/2 = 100 m2 a) Continuous quiescent sedimentation, 0.125 kg/m3 b) Complete-mix sedimentation, 0.167 kg/m3 c) Plug flow vertical mixing sedimentation. 0.152 kg/m3 Comp lete -m ix sedimenta tion Plug flo w vertica l mixing sedimenta tion