Quantum LP Short
advertisement
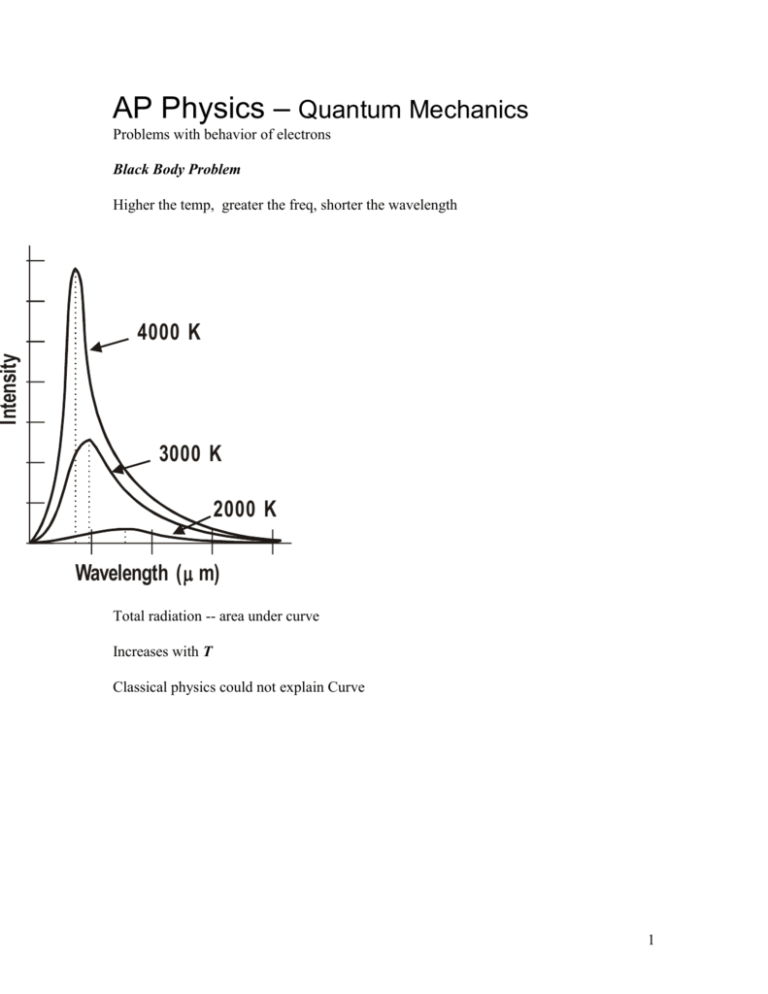
AP Physics – Quantum Mechanics Problems with behavior of electrons Black Body Problem Higher the temp, greater the freq, shorter the wavelength Intensity 4000 K 3000 K 2000 K Wavelength ( m) Total radiation -- area under curve Increases with T Classical physics could not explain Curve 1 Intensity Classical Theory Planck’s Theory Wavelength Classical mechanics works -- long wavelengths Classical mechanics predicted E would approach infinity as wavelength approached zero E hf Max Planck h 6.626 x 1034 J s h 4.14 1015 eV s What energy is carried by a quantum (photon) of electromagnetic radiation that has a frequency of 1.55 x 1017 Hz? E hf 1 6.63 x 1034 J s 1.55 x 1017 s E 10.3 x 1017 J 1.03 x 1016 J Energy as function of wavelength: 2 cf f c E hf E E hc hc Photoelectric Effect Light incident on metals – electrons get emitted Work Function Energy that binds electron to metal Depends on metal KMax hf Photon’s energy less work function Table 27.1 pg 890 Look at wavelength c c f K Max hc f Equation on AP test Values for hc terms: hc 1.99 x 1025 J m hc 1.24 x 103 eV nm These values are on AP test 3 What is the velocity of a photoelectron that has been liberated from a zinc metal surface by a photon that has a wavelength of 275 nm? work function for zinc is 4.31 eV K Max hc 1.24 x 103 eV nm 4.31 eV 275 nm K Max 0.00451 x 103 eV 4.31 eV KMax 4.51 eV 4.31 eV 0.200 eV 1.6 x 1019 J 19 K Max 0.200 eV 0.32 x 10 J 1 eV 1 K mv 2 2 v 2K m 2 20 kg m 2 3.2 x 10 s 2 v 9.11 x 1031 kg m2 v 7.025 x 10 s2 10 3.2 x 1020 J m2 0.7025 x 10 2 s 2.65 x 105 11 m s Photoelectric Lab Setup: 4 Emitter Plate at neg potential Light strikes emitter C E e A V Variable power supply Electrons emitted Electrons travel to Collector Vary wavelength: intensity constant, Get current curve Current 0 photo-electric threshold wavelength Current Depends on wavelength Increase V -- max current Even at zero potential get some current 0 Wavelength Make Collector negative 5 -- Collector Repels electrons -- Current drops to low value High Intensity Current Low Intensity VS Applied Voltage V less than or equal to Vs no electrons reach C All electrons get turned away Vs Stopping potential current is zero no electrons reach Collector Stopping potential independent of light intensity Max K is energy gained in field (PE: U E qV SO KMax qVs KMax independent of intensity! Einstein explained in 1905 (Noble Prize) Electrons bound to metal Photon gives all its energy to single electron Electron gains energy and is liberated 6 Energy gained exceeds the energy that binds it to the metal graph of frequency VS. kinetic energy Has straight line. KE fC cutoff frequency Slope of graph is h Cutoff f fc Frequency -minimum frequency that will generate photoelectrons minimum frequency is when kinetic energy = zero. Set KMax = 0 K Max hf fC hf f h h Cutoff Wavelength Set KMax = 0 7 –photon E can just overcome work function K Max 0 hc hc hc hc C hc be prepared to derive these equations on AP test What is the cutoff wavelength for a copper metal surface? C hc 1.24 x 103 eV nm 4.70 eV 0.264 x 103 nm 264 nm wavelength is smaller than visible light Finding Work Function: - solve for Set KMax = 0 Use cutoff wavelength K Max hc C 0 hc C hc C hc C Also can find as function of cutoff frequency. KMax hf 0 hf hf hfC 8 500.0 nm light is incident on a metal surface. The stopping potential is found to be 0.440 V. (a) Find the work function for this material and (b) the longest wavelength that will eject electrons from the metal. (a) work function: K Max hc qVs hc K Max qVs hc qVs 1.24 x 103 eV nm e 0.440 V 0.00248 x 103 eV 0.440 eV 500 nm 2.48 eV 0.440 eV 2.04 eV (b) longest wavelength C hc 1.24 x 103 eV nm 204 eV 0.00608 x 103 nm 608 nm Classical Mechanics or wave theory cannot explain: No electrons emitted if light freq falls below cutoff freq, fC Max K independent of light intensity Electrons emitted almost instantaneously KEMax increases with increasing freq as it is function of hf Happens so fast because it is a one to one photon/electron deal 9 AP Test: A sodium photoelectric surface with work function 2.3 eV is illuminated by electromagnetic radiation and emits electrons. The electrons travel toward a negatively charged cathode and complete the circuit shown below. The potential difference supplied by the power supply is increased, and when it reaches 4.5 V, no electrons reach the cathode. (a) For the electrons emitted from the sodium surface, calculate the following. i. The maximum kinetic energy. KMax qVs K Max 1.6 x 1019 C 4.5 V 7.2 x 1019 J ii. The speed at this maximum kinetic energy. 1 K mv 2 2 v 2K m 2 19 kg m 2 7.2 x 10 2 s 31 9.11 x 10 Kg 1.26 x 106 m s (b) Calculate the wavelength of the radiation that is incident on the sodium surface. K Max hc hc KEMax K Max hc K Max hc 1.24 x 103 eV nm 4.5 eV 2.3 eV hc K Max 0.182 x 103 nm 182 nm (c) Calculate the minimum frequency of light that will cause photoemission from this sodium surface. KMax hf KMax 0 for minimum Freq 0 hf hf f h 10 f 2.3 eV 4.14 x 1015 eV s 0.555 x 1015 Hz 5.55 x 1014 Hz Stopping Potential versus frequency Stopping potential is function of frequency Higher frequency higher VS Vs f fc intercept on x axis is stopping potential for cutoff frequency. At fC - total E = E hfC E qVS E eVS This energy = energy of photon, so: hf eVs solve for h/e h Vs e f 11 h/e is slope of the graph AP Test Item: In a photoelectric experiment, light is incident on a metal surface. Electrons are ejected from the surface, producing a current in a circuit. A reverse potential is applied in the circuit and adjusted until the current drops to zero. That potential at which the current drops to zero is called the stopping potential. The data obtained for a range of frequencies are graphed below. a. For a frequency of light that has a stopping potential of 3.0 volts, what is the maximum kinetic energy of the ejected photoelectrons? K Max qVs e 3.0 V 3.0 eV b. From the graph and the value of the electron charge, determine an experimental value for Planck's constant. Slope of graph is h 2V 0V e 10 51014 Hz h e h 1.6 x 1019 C 2 V 0 V 10 51014 Hz 12 3.2 x 1019 J h 1 5 x 1014 s h V2 V1 e f 2 f1 h e2 V 1 5 x 1014 s 0.64 x 1033 J s 6.4 x 1034 J s 2V 0V 10 51014 Hz e2 V 1 5 x 1014 s h 0.40 x 1014 eV s or 4.0 x 1015 eV s c. From the graph, determine the work function for the metal. 1 4.14 1015 eV s 5.0 x 1014 20.7 101 eV 2.07 eV g s h raph of straight line y mx b Vs f b eVs hf eb e K Max hf eb But eVs is KMax hfC so y intercept is work function times e This is 2.0 eV d. On the axes above, draw the expected graph for a different metal surface with a threshold frequency of 6.0 x 1014 hertz. 13 Photons and Power: Given power of light source and wavelength Can calculate number of photons per second. An LED produces 685 nm light at a power rating of 1.25 x 10-7 W. Find the number of photons emitted per second. P E t E Pt let t = 1 second 1.25 x 107 solve for E J 1 s 1.25 x 107 J s Energy of one photon: E hc 1.24 x 103 eV nm 685 nm 1.25 x 10 7 1 eV J 19 1.6 x 10 J 0.00181 x 103 eV 1.81 eV 1 photon 12 0.432 x 10 photon 1.81 eV 4.32 x 1011 photon 14








