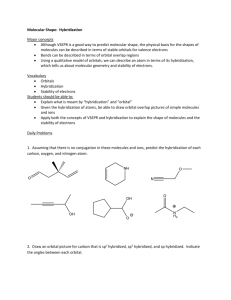
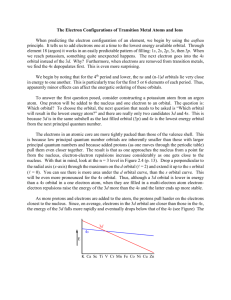
Modern Atom Theory
advertisement
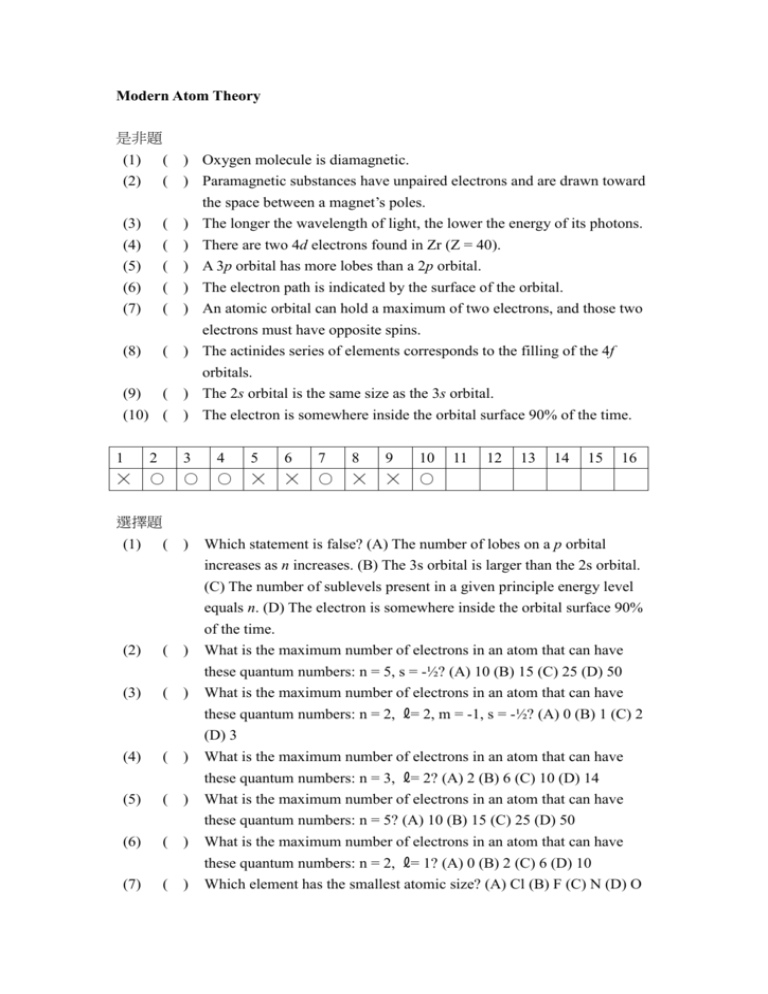
Modern Atom Theory 是非題 (1) (2) ( ) Oxygen molecule is diamagnetic. ( ) Paramagnetic substances have unpaired electrons and are drawn toward the space between a magnet’s poles. ( ) The longer the wavelength of light, the lower the energy of its photons. ( ) There are two 4d electrons found in Zr (Z = 40). ( ) A 3p orbital has more lobes than a 2p orbital. ( ) The electron path is indicated by the surface of the orbital. ( ) An atomic orbital can hold a maximum of two electrons, and those two (3) (4) (5) (6) (7) electrons must have opposite spins. ( ) The actinides series of elements corresponds to the filling of the 4f orbitals. (8) (9) ( ) The 2s orbital is the same size as the 3s orbital. (10) ( ) The electron is somewhere inside the orbital surface 90% of the time. 1 2 3 4 5 6 7 8 9 10 ☓ ○ ○ ○ ☓ ☓ ○ ☓ ☓ ○ 11 12 13 14 15 16 選擇題 (1) ( ) (2) ( ) (3) ( ) Which statement is false? (A) The number of lobes on a p orbital increases as n increases. (B) The 3s orbital is larger than the 2s orbital. (C) The number of sublevels present in a given principle energy level equals n. (D) The electron is somewhere inside the orbital surface 90% of the time. What is the maximum number of electrons in an atom that can have these quantum numbers: n = 5, s = -½? (A) 10 (B) 15 (C) 25 (D) 50 What is the maximum number of electrons in an atom that can have ( ) these quantum numbers: n = 2, ℓ= 2, m = -1, s = -½? (A) 0 (B) 1 (C) 2 (D) 3 What is the maximum number of electrons in an atom that can have (4) (5) ( ) (6) ( ) these quantum numbers: n = 3, ℓ= 2? (A) 2 (B) 6 (C) 10 (D) 14 What is the maximum number of electrons in an atom that can have these quantum numbers: n = 5? (A) 10 (B) 15 (C) 25 (D) 50 What is the maximum number of electrons in an atom that can have ( ) these quantum numbers: n = 2, ℓ= 1? (A) 0 (B) 2 (C) 6 (D) 10 Which element has the smallest atomic size? (A) Cl (B) F (C) N (D) O (7) (8) ( ) (9) ( ) (10) ( ) (11) ( ) (12) ( ) (13) ( ) (14) ( ) Which element would be expected to have the highest ionization energy? (A) Mg (B) Na (C) S (D) Si How many 4d electrons are found in Zr (Z = 40)? (A) 2 (B) 4 (C) 6 (D) 8 The correct configuration for Cu (Z = 29) is (A) [Ar]3d6 (B) [Ar]4s13d5 (C) [Ar]4s23d4 (D) none of these. The electron configuration of chromium is (A) [Ar]4s13d5 (B) [Ar]4s23d4 (C) [Ar]4s23d14p3 (D) [Ar]4s33d3. How many valence electrons does Krypton (Z = 36) possess? (A) 2 (B) 4 (C) 6 (D) 8 The lantnanide series of elements corresponds to the filling of the (A) 3f (B) 4f (C) 5f (D) 6f orbitals. Which of the following orbital designation is not correct? (A) 2d (B) 2p (C) 4d (D) 4f 1 2 3 4 5 6 7 8 9 10 11 12 13 14 A C A C D C B C A D A D B A 15 問答、計算題 (1) Write the full electron configuration (1s22s2, etc) for each of the following elements? (a) chlorine, Z = 17 (1) (b) copper, Z = 29 (a) 1s22s22p63s23p5 or [Ne]3s23p5 (b) 1s22s22p63s23p64s13d10 or [Ar] 4s13d10 16