3.9, 3.10, 3.29, 3.30, 3.42, 3.47, 3.49
advertisement
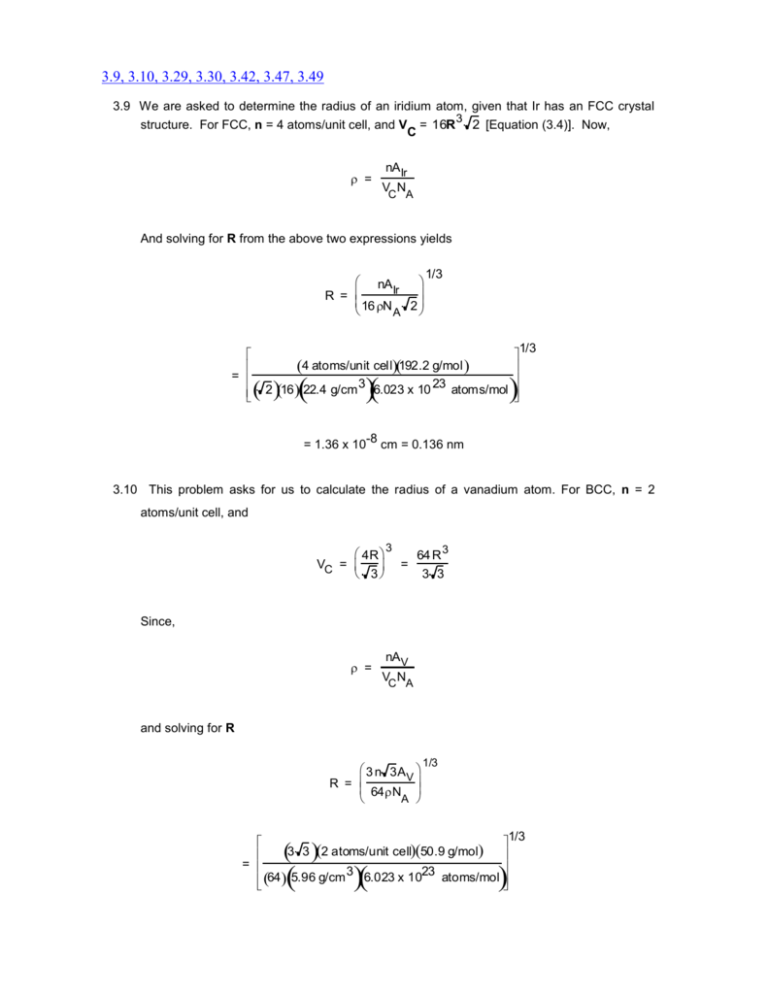
3.9, 3.10, 3.29, 3.30, 3.42, 3.47, 3.49 3.9 We are asked to determine the radius of an iridium atom, given that Ir has an FCC crystal structure. For FCC, n = 4 atoms/unit cell, and V = 16R 3 2 [Equation (3.4)]. Now, C = nA Ir VC NA And solving for R from the above two expressions yields 1/3 nA Ir R = 16 N 2 A 4 atoms/unit cell192.2 g/mol = 3 23 2 16 22.4 g/cm 6.023 x 10 atoms/mol = 1.36 x 10 -8 1/3 cm = 0.136 nm 3.10 This problem asks for us to calculate the radius of a vanadium atom. For BCC, n = 2 atoms/unit cell, and 3 4R VC = 3 3 = 64 R 3 3 Since, = nA V VC NA and solving for R 1/3 3 n 3A V R = 64N A 1/3 3 3 2 atoms/unit cell 50.9 g/mol = 3 23 64 5.96 g/cm 6.023 x 10 atoms/mol = 1.32 x 10 -8 cm = 0.132 nm 3.29 (a) This portion of the problem asks that a [0 1 1] direction be drawn within a monoclinic unit cell (a ≠ b ≠ c, and = = 90 ≠ ). One such unit cell with its origin at point O is sketched below. For this direction, there is no projection along the x-axis since the first index is zero; thus, the direction lies in the y-z plane. We next move from the origin along the minus y-axis b units (from point O to point R). Since the final index is a one, move from point R parallel to the z-axis, c units (to point P). Thus, the [0 1 1] direction corresponds to the vector passing from the origin to point P, as indicated in the figure. (b) A (002) plane is drawn within the monoclinic cell shown below. We first remove the parentheses and take the reciprocals of the indices; this gives , , and 1/2. Thus, the (002) plane parallels both x- and y-axes, and intercepts the z-axis at c/2, as indicated in the drawing. 3.30 (a) We are asked for the indices of the two directions sketched in the figure. For direction 1, the projection on the x-axis is zero (since it lies in the y-z plane), while projections on the y- and z-axes are b/2 and c, respectively. This is an [012] direction as indicated in the summary below Projections x y z 0a b/2 c 0 1/2 1 0 1 2 Projections in terms of a, b, and c Reduction to integers [012] Enclosure Direction 2 is [11 2] as summarized below. Projections x y z a/2 b/2 -c 1/2 1/2 -1 1 1 -2 Projections in terms of a, b, and c Reduction to integers Enclosure [11 2] (b) This part of the problem calls for the indices of the two planes which are drawn in the sketch. Plane 1 is an (020) plane. The determination of its indices is summarized below. x y z a b/2 c Intercepts in terms of a, b, and c 1/2 Reciprocals of intercepts 0 2 0 Intercepts (020) Enclosure Plane 2 is a (22 1) plane, as summarized below. x y z Intercepts a/2 -b/2 c Intercepts in terms of a, b, and c 1/2 -1/2 1 2 -2 1 Reciprocals of intercepts 3.42 (a) The atomic packing of the (100) plane for the FCC crystal structure is called for. An FCC unit cell, its (100) plane, and the atomic packing of this plane are indicated below. (b) For this part of the problem we are to show the atomic packing of the (111) plane for the BCC crystal structure. A BCC unit cell, its (111) plane, and the atomic packing of this plane are indicated below. 3.47 (a) In the figure below is shown a (100) plane for an FCC unit cell. For this (100) plane there is one atom at each of the four cube corners, each of which is shared with four adjacent unit cells, while the center atom lies entirely within the unit cell. Thus, there is the equivalence of 2 atoms associated with this FCC (100) plane. The planar section represented in the above figure is a square, wherein the side lengths are equal to the unit cell edge length, 2R 2 [Equation (3.1)]; and, thus, the area of this square is just 2R 22 = 8R2. Hence, the planar density for this (100) plane is just PD100 = number of atoms centered on (100) plane area of (100) plane 2 atoms 8 R2 1 4R 2 That portion of an FCC (111) plane contained within a unit cell is shown below. There are six atoms whose centers lie on this plane, which are labeled A through F. Onesixth of each of atoms A, D, and F are associated with this plane (yielding an equivalence of one-half atom), with one-half of each of atoms B, C, and E (or an equivalence of one and one-half atoms) for a total equivalence of two atoms. Now, the area of the triangle shown in the above figure is equal to one-half of the product of the base length and the height, h. If we consider half of the triangle, then (2R)2 h2 (4R)2 which leads to h = 2 R 3 . Thus, the area is equal to Area 4R(h) (4R) 2R 3 2 4R 2 2 3 And, thus, the planar density is number of atoms centered on (111) plane area of (111) plane PD111 = 2 atoms 4R 2 3 1 2R2 3 (b) From the table inside the front cover, the atomic radius for aluminum is 0.143 nm. Therefore, the planar density for the (100) plane is PD100 (Al) 1 4 R2 1 4(0.143 nm)2 12.23 nm 2 1.223 x 10 17 2 m While for the (111) plane PD111(Al) 1 2R2 3 1 2 3 (0.143 nm) 2 14.12 nm 2 1.412 x 10 17 m2 3.49 (a) A (0001) plane for an HCP unit cell is show below. Each of the 6 perimeter atoms in this plane is shared with three other unit cells, whereas the center atom is shared with no other unit cells; this gives rise to three equivalent atoms belonging to this plane. In terms of the atomic radius R, the area of each of the 6 equilateral triangles that have been drawn is R2 3 , or the total area of the plane shown is 6R 2 3 . And the planar density for this (0001) plane is equal to PD0001 = number of atoms centered on (0001) plane area of (0001) plane (b) 3 atoms 6R2 3 1 2R2 3 From the table inside the front cover, the atomic radius for titanium is 0.145 nm. Therefore, the planar density for the (0001) plane is PD0001(Ti) 1 2R2 3 1 2 3 (0.145 nm) 2 13.73 nm 2 1.373 x 10 17 m 2